In 2009 a research group in Japan isolated a novel class of polyketides, subsequently named indoxamycins, from saline cultures of marine-derived actinomycetes. [1]
Within this family, indoxamycins A and F have been shown to display growth inhibition against HT-29 tumor cell lines (IC50 = 0.59 mm and 0.31 mm, respectively). Their biological activity in conjunction with the highly congested and stereochemically dense core render the indoxamycins notable as targets for synthetic studies. The absolute and relative stereochemistry of indoxamycins was originally assigned based on a combination of one- and two-dimensional NMR experiments and CD studies.
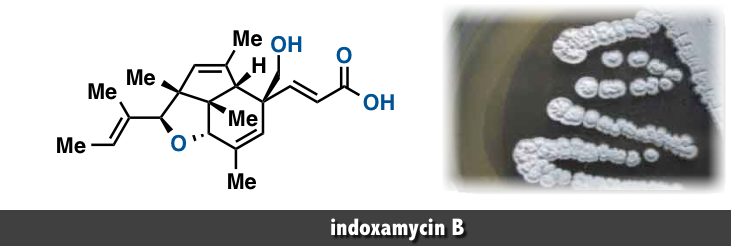
The indoxamycins skeleton consists of an unprecedented [5,5,6] tricyclic cage-like carbon framework and two side chains having a trisubstituted olefin and an unsaturated carboxylic acid, respectively. The core structure features six contiguous stereogenic centers, of which three are quaternary, including two vicinal carbon atoms embedded in a sterically congested tetrahydrofuran subunit .
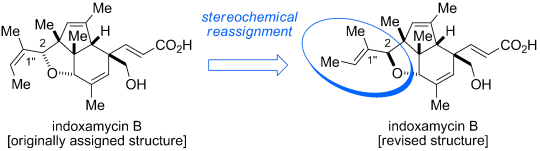
In 2012, Carreira and co-workers reported an elegant total synthesis of rac-indoxamycin B, which led to a structural reassignment of the relative configuration at the C2 position and the geometry of the trisubstituted alkene in the side chain.[2] Next year, Ding and coworkers have accomplished total synthesis of indoxamycins A, C, and F in their racemic and enantiomerically pure forms. [3]
- References
[1] “Indoxamycins A−F. Cytotoxic Tricycklic Polypropionates from a Marine-Derived Actinomycete”
Sato, S. Iwata, F.; Mukai, T.; Yamada, S. Takeo, J.; Abe, A. Kawahara, H. J. Org. Chem. 2009, 74, 5502. DOI: 10.1021/jo900667j
Six antitumor antibiotics of a new structure class, indoxamycins A−F (1−6), were isolated from a saline culture group of marine-derived actinomyces whose strains showed approximately 96% sequence homology of 16S rDNA with the family streptomycetaceae. The structures of these indoxamycins, which are unusual polyketides composed of six consecutive chiral centers, were assigned by combined spectral and chemical methods. In feeding experiments using a stable isotope label, indoxamycin A was assembled from propionate units initially forming the “aglycon” pentamethyl indeno furan. The discovery of these unprecedented compounds from marine-derived actinomycetes, a low gene homology genus, offers a significant opportunity for drug discovery.
[2] “Total Synthesis and Stereochemical Reassignment of (+-)-Indoxamycin B”
Jeker, O. F.; Carreira, E. M. Angew. Chem. Int. Ed. 2012, 51, 3474–3477.
Revised version: The first total synthesis of indoxamycin B leads to a stereochemical reassignment of the natural product (see picture). The synthetic route features an efficient carboannulation sequence to rapidly access the dihydroindenone system. Moreover, a series of AuI-catalyzed transformations served in the construction of the sterically congested core framework.
[3] “Divergent Total Synthesis of Indoxamycins A, C, and F”
He, C.; Zhu, C.; Dai, Z.; Tseng, C.-C.; Ding, H. Angew. Chem. Int. Ed. 2013, 52, 13256–13260. DOI: 10.1002/anie.201307426
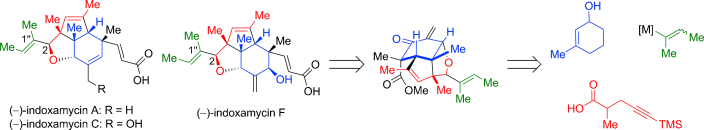
The concise and divergent: total synthesis of (−)-indoxamycins A, C, and F has been completed for the first time by using a tricyclic enone as the common late-stage intermediate. The key steps of the strategy are based on an Ireland–Claisen rearrangement, a stereodivergent reductive 1,6-enyne cyclization, and a tandem 1,2-addition/oxa-Michael/methylenation reaction.
- Related Books
[amazonjs asin=”1617796239″ locale=”US” title=”Natural Products Isolation (Methods in Molecular Biology)”][amazonjs asin=”B00DF3RT5S” locale=”US” title=”Heterocycles in Natural Product Synthesis”]