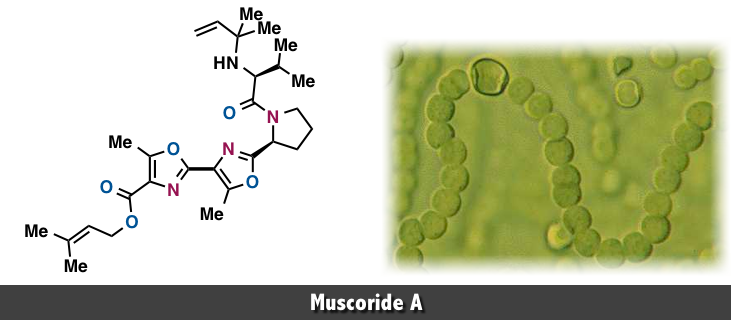
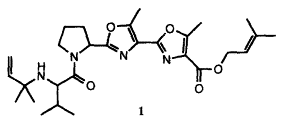
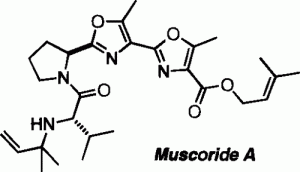
Muscoride A, a natural product with antibacterial activity, was isolated by Sakakibara in 1995,[1] and its total synthesis has been accomplished by Wipf,[2] Pattenden,[3], Ciufolini[4], and Itami, Yamaguchi[5].
-
References
[1] “Muscoride A: A new oxazole peptide alkaloid from freshwater cyanobacterium Nostoc muscorum”
Nagatsu, A.; Kajitani, H.; Sakakibara, J. Tetrahedron Lett. 1995, 36, 4097. DOI: 10.1016/0040-4039(95)00724-Q
The novel oxazole peptide alkaloid, muscoride A (1), was isolated from terrestrial freshwater cyanobacterium Nostoc muscorum. The structure of 1 was elucidated by spectral methods. Muscoride A (1) is the first compound possessing N-(2-methyl-3-buten-2-yl)valine and two contiguous methyloxazoles.
[2] “Total Synthesis of (−)-Muscoride A”
Wipf, P.; Venkatraman, S. J. Org. Chem. 1996, 61, 6517–6522. DOI: 10.1021/jo960891m
The recently isolated cyanobacterium metabolite muscoride A was synthesized in 15 steps and in 4.3% overall yield. Novel structural features of this peptide antibiotic include the presence of a threonine-derived bioxazole core and an N-(1,1-dimethyl)allyl (“reverse prenyl”) valine residue. In the context of our synthesis, efficient new strategies for the preparation of these segments were developed. The synthesis of two epimers of muscoride A allowed the unambiguous assignment of the relative and absolute configuration of the natural product by NMR and optical rotation analyses.
[3] “Total Synthesis of (-)-Muscoride A: A Novel Bis-Oxazole Based Alkaloid from the Cyanobacterium Nostoc muscorum”
Muir, J. C. Pattenden, C, Thomas, R. M. Synthesis 1998, 1998, 613–618. DOI: 10.1055/s-1998-5934
A convergent total synthesis of the substituted bis- oxazole (–)-muscoride A (1), isolated from a cyanobacterium, is described based on coupling of the N-functionalised “reverse pre- nyl” dipeptide acid to the threonine-based oxazole, followed by elaboration of the tetrapeptide, via the oxazoline-oxazole and the bis-oxazole ester.
[4] “Iterative Oxazole Assembly via α-Chloroglycinates: Total Synthesis of (−)-Muscoride A”
Coqueron, P.-Y.; Didier, C.; Ciufolini, M. A. Angew. Chem. Int. Ed. 2003, 42, 1411–1414. DOI: 10.1002/anie.200390363
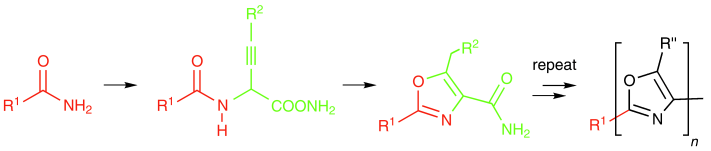
An efficient iterative synthesis of oxazoles involves the cyclization of 2-alkynyl glycinates (see scheme) formed from the reaction of 1-alkynyl aluminum reagents with α-chloroglycinates. This approach was used in the total synthesis of the bisoxazole natural product, (−)-muscoride A.
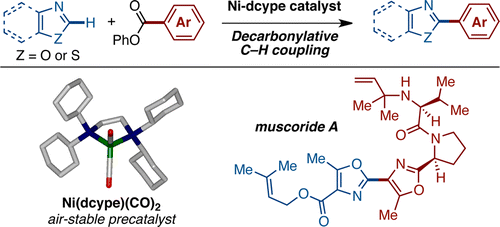
[5] “Decarbonylative C−H Coupling of Azoles and Aryl Esters: Unprecedented Nickel Catalysis and Application to the Synthesis of Muscoride A”
Amaike, K.; Muto, K.; Yamaguchi, J.; Itami, K. J. Am. Chem. Soc. 2012, 134, 13573–13576. DOI:10.1021/ja306062c
A nickel-catalyzed decarbonylative C–H biaryl coupling of azoles and aryl esters is described. The newly developed catalytic system does not require the use of expensive metal catalysts or silver- or copper-based stoichiometric oxidants. We have successfully applied this new C–H arylation reaction to a convergent formal synthesis of muscoride A.
- Related Books
[amazonjs asin=”B005UQCYWG” locale=”US” title=”Medicinal Natural Products: A Biosynthetic Approach”][amazonjs asin=”3659228893″ locale=”US” title=”Antimicrobial Azole Heterocycles: A Study: 1,3,4-oxadiazole: A Scaffold Of Antimicrobial Potential”]