- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
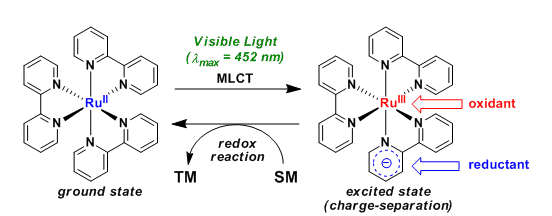
Transition metal complexes such as ruthenium(II) polypyridyl and iridium(III) phenylpyridyl are known to function as photoredox catalysts under irradiated conditions with visible light (λ=400-700 nm).
Because these systems do not rely on high energy ultraviolet light, side reactions can be expected to be minimized. The easy operation and environmental friendliness are also positive aspects from the perspective of green chemistry.
The utilization of visible light as an energy source itself is drawing an attention as an important technological paradigm.
-
General References
<Ru(bpy)32+>
- Kalyanasundaram, K. Coord. Chem. Rev. 1982, 46, 159. doi:10.1016/0010-8545(82)85003-0
- Julis, V.; Balzani, V. Coord. Chem. Rev. 1988, 84, 85. doi:10.1016/0010-8545(88)80032-8
- Broomhead, J. A.; Young, C. G. Inorg. Synth. 1990, 28, 338. doi:10.1002/9780470132593.ch86
<Ir(ppy)2(dtb-bpy)+>
- Bernhard, S.; Malliaras, G. G. et al. J. Am. Chem. Soc. 2004, 126, 2763. DOI: 10.1021/ja0345221
<Reviews of Application to Organic Synthesis>
- Zeitler, K. Angew. Chem. Int. Ed. 2009, 48, 9785. doi:10.1002/anie.200904056
- Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527. doi:10.1038/nchem.687
- Teply, F. Collect. Czech. Chem. Commun. 2011, 76, 859. doi:10.1135/cccc2011078
- Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102. doi:10.1039/b913880n
- Tucker, J. Q.; Stephenson, C. R. J. J. Org. Chem. 2012, 77, 1617. DOI: 10.1021/jo202538x
- Allen, A. E.; MacMillan, D. W. C. Chem. Sci. 2012, 3, 633. DOI: 10.1039/c2sc00907b
- Shi, L.; Xia, W. Chem. Soc. Rev. 2012, DOI: 10.1039/c2cs35203f
- Ravelli, D>; Fagnoni, M. ChemCatChem 2012, 4, 169. DOI: 10.1002/cctc.201100363
-
Reaction Mechanism
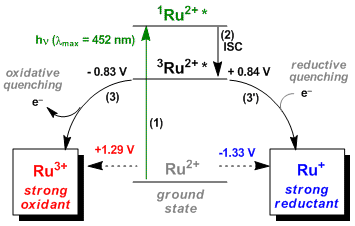
(Extracted from Collect. Czech. Chem. Commun. 2011, 76, 859 and used with modification)
The mechanism of how Ru(bpy)32+ is thought to operate is depicted above with basic parameters (vs. SCE).
(1) Excitation from the ground state by the energy of visible light.
(2) Intersystem crossing (ISC) to the triplet state (3MLCT).
(3)(3’) In the presence of a sacrificial oxidant or reductant, quenching leads to the formation of oxidizing species (Ru3+) or reductive species (Ru+), respectively.
(4) Ru3+ or Ru+ promotes the chemical reaction and returns to the ground state.
Therefore, the ruthenium can act as either an oxidant or reductant depending on the type of substrates and reagents.
Fine-tuning of redox potential is also possible by modifying the ligand’s electronic property.
-
Examples
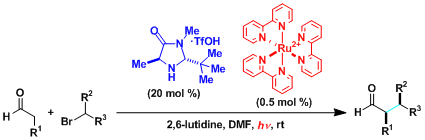
Merging of photoredox catalysis with organocatalysis.[1]
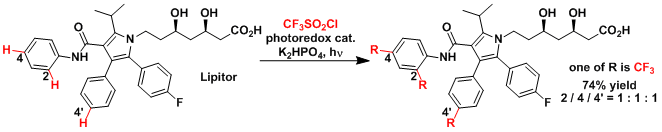
The late-stage trifluoromethylation of Lipitor.[2]
-
Experimental Tips
-
References
- Nicewicz, D. A.; MacMillan, D. A. Science 2008, 322, 77. doi:10.1126/science.1161976
- Nagib, D. A.; MacMillan, D. W. C. Nature 2011, 480, 224. doi:10.1038/nature10647
< Other Representative Report in Organic Synthesis> - Ischay, M. A.; Anzovino, M. E.; Du, J.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 12886. doi:10.1021/ja805387f
- Dai, C.; Narayanam, J. M. R.; Stephenson, C. R. J. Nat. Chem. 2011, 3, 140. doi:10.1038/nchem.949
- Neumann, M.; Fuldner, S.; Konig, B.; Zeitler, K. Angew. Chem. Int. Ed. 2011, 50, 951. DOI: 10.1002/anie.201002992
- McNally, A.; Prier, C. K.; MacMillan, D. W. C Science 2011, 334, 1114. DOI:10.1126/science.1213920
-
Related Reactions
-
Related Books
-
External Links