Samuel J. Danishefsky (born 1936, Bayonne, NJ) is synthetic organic chemist who accomplished in natural products total synthesis and pioneer for chemical synthesis of carbohydrates for the development of anticancer vaccines.
Currently, Danishefsky serves as Cenntenial Professor of Chemistry at Columbia University, where he conducts research as well as at the Sloan-Kettering Cancer Center’s molecular pharmacology and chemistry program. He is one of the world’s pre-eminent medicinal chemists, recognized in particular for his work on the synthesis of anti-tumor agents derived from natural products.
Remarkably, many chemists appeared from his followers. Former students and postdocs include:
Peter Seeberger, Dalibor Sames, Eric Sorensen, David Gin, Cholbum Lee, Matt Shair, Jon Clardy, Rob Coleman, Masahiro Hirama, Takeshi Kitihara, Raymond Funk, William Pearson, Jeffrey Aube, James Panek, Robert Zamboni, Robert Volkman, J. T. Link, Mike Silvestri, Mike Bednarski, Margaret Chu Moyer, R.C. A. Isaacs, Roger Ruggeri, Ray Cvetovich, Dave Askin, Gayle Schulte, Dirk Trauner, Robert Coleman, Martin Maier, Gary Sulikowski, David Berkowitz, Frank McDonald, Ohyun Kwon, Dionicio Siegel, Masayuki Inoue, Tristan Lambert, Ed Turos, Randy Halcomb, Jaqueline Gervay, Jon Njardarson and Alison Frontier
-
Education and Experiences
1956 B. S. Yeshiva University
1962 Ph.D. in chemistry, Harvard University (Prof. Peter Yates)
1962 a National Institutes of Health postdoctoral fellowship, Columbia University (Prof. Gilbert Stork)
1963 Professor at the University of Pittsburgh
1980 Professor at Yale University
1991- Memorial Sloan-Kettering Cancer Center as director of the Laboratory for Cancer Research
-
Awards and Honors
1996 Tetrahedron Prize in Chemistry
1996, The Wolf Prize in Chemistry (with Professor Gilbert Stork)
1997 the New York City Mayor’s Award for Science and Technology
1997 the Claude S. Hudson Award in Carbohydrate Chemistry from the American Chemical Society
1998 the Arthur C. Cope Medal
1999 the Nichols Medal
1998 the Ehrlich award from the French Pharmaceutical Society
2006 National Academy of Science Award for Chemical Sciences
2006 the Benjamin Franklin Medal in Chemistry
2006 the Bristol-Myers Squibb Distinguished Lifetime Achievement Award in Organic Synthesis
-
Research
His research includes the synthesis of substances of complex structure with interesting biological activity; new synthetic strategies of applicability not only to such target systems, but to general problems in synthesis; degree of stereochemical control which can be exercised; the understanding of the origins of diastereofacial and topographic selectivity are of continuing interest.
In 1974, the Danishefsky group employed for the first time its now famous diene (Kitahara-Danishefsky diene) as well as the then unknown reaction of an enolate (or silyl enol ether) with the Eschenmoser salt.
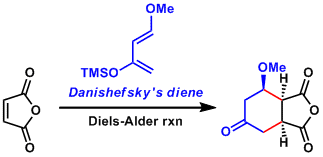
Danishefsky’s elegant and first ever synthesis of Vernolepin cemented his place as one of the top synthetic chemists in the world.[2]
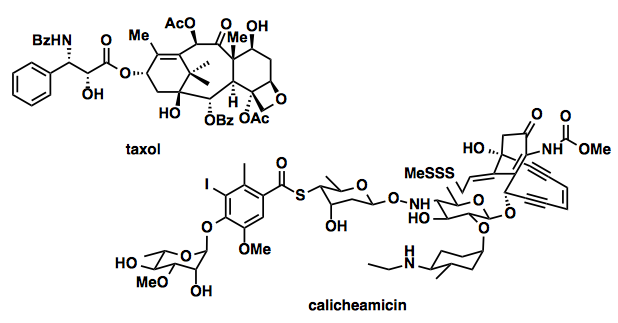
Other examples for the elegant total synthesis are: taxol,[3] calicheamicin,[4] dynemicin,[5] staurosporine, FR-900482 (a complex mitomycinoid), tryprostatin, N-acetylardeemin, a human breast tumor antigen, a human gastric cancer antigen, asparagine linked glycopeptides (ABO blood group antigens).
-
References
[1] Danishefsky, S.; Kitahara, T. J. Am. Chem. Soc. 1974, 96, 7807. DOI: 10.1021/ja00832a031
[2] Danishefsky, S.; Kitahara, T.; Schuda, P. F.; Etheredge, S. J. J. Am. Chem. Soc. 1976, 98, 3028. DOI: 10.1021/ja00426a066
[3] Danishefsky, S. J.; Masters, J. J.; Link, J. T.; Young, W. B.; Snyder, L. B.; Magee, T. V.; Jung, D. K.; Isaacs, R. C. A.; Bornmann, W. G.; Alaimo, C. A.; Coburn, C. A.; DiGrandi, M. J. J. Am. Chem. Soc. 1996, 118, 2843. DOI:10.1021/ja952692a
[4] Cabal, M. P.; Coleman, R. S.; Danishefsky, S. J. J. Am. Chem. Soc. 1990, 112, 3253. DOI: 10.1021/ja00164a079
[5] Shair, M. D.; Yoon, T. Y.; Mosny, K. K.; Chou, T. C. J. Am. Chem. Soc. 1996, 118, 9509. DOI: 10.1021/ja960040w
[6]Perspective :”Pattern Recognition in Retrosynthetic Analysis: Snapshots in Total Synthesis”
Wilson, R. M.; Danishefsky, S. J. J. Org. Chem. 2007, 72, 4293. DOI:10.1021/jo070871s
In this Perspective, the value of small molecule natural products (SMNPs) in the discovery of active biological agents is discussed. The usefulness of the natural products-based method of potential pharma discovery is much augmented by the capacities of chemical synthesis. The great advances in synthetic methodology allow for major editing of the natural product in the hopes of optimizing potency and therapeutic index. As a consequence of the enormous increase in the power of multistep chemical synthesis, one can now approach structures of previously impractical complexity. In constructing a plan for a multistep synthesis, two complementary thought styles are often encountered. One is the traditional and extremely powerful concept of prioritized strategic bond disconnections. The other, which we term “pattern recognition,” involves the identification of moieties within the target, which are associated with reliable chemistry, and can serve to facilitate progress to the target. Recognition of such targets may require substantial recasting of the target structure to connect it to well-established types of transformations. Some of our older ventures, where ideas about pattern recognition were first being fashioned and used productively, are revisited. In addition, we provide snapshots of recently achieved total syntheses of SMNPs of novel biological potential. These vignettes serve to harmonize insights occasioned by pattern recognition, in concert with transformations enabled by the enormous growth in the power of synthesis.
[7] Perspective : “Small Molecule Natural Products in the Discovery of Therapeutic Agents: The Synthesis Connection”
Wilson, R. M.; Danishefsky, S. J. J. Org. Chem. 2006, 71, 8329. DOI:10.1021/jo0610053
Natural products have been a rich source of agents of value in medicine. They have also inspired, at various levels, the fashioning of nonnatural agents of pharmaceutical import. Hitherto, these nonnatural derivatives have been primarily synthesized by manipulating the natural product. As a consequence of major innovations in the subscience of synthetic methodology, the capacity of synthesis to deal with molecules of considerable complexity has increased dramatically. In this paper, we show by example some total syntheses which draw from strategy-enabling advances in methodology. Moreover, we show how these capabilities can be used to discover and develop new agents of potential pharmaceutical value without recourse to the natural product itself.
-
Related Links
Samuel Danishefsky
Samuel Danishefsky
Samuel Danishefsky - Wikipedia
Danishefsky Taxol total synthesis - Wikipedia
-
Photo Gallery
-
Related Books
[amazonjs asin=”184973965X” locale=”US” title=”Carbohydrate Chemistry: Volume 40 (Specialist Periodical Reports)”][amazonjs asin=”3527319646″ locale=”US” title=”Design and Strategy in Organic Synthesis”]