- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The Vilsmeier-Haack reaction is a common method to introduce a formyl group into electron rich aromatic compounds. It is also applicable to electron rich alkenes. The procedure is simple and the reaction conditions are not particularly harsh either.
-
General References
・Vilsmeier, A.; Haack, A. Ber. 1927, 60, 119.
・Jackson, W. G. et al. J. Am. Chem. Soc. 1991, 103, 533. DOI: 10.1021/ja00393a008
・Meth-Cohn, O, Comprehensive Organic Synthesis 1991, 2, 777.
・Jones, G. Org. React. 2000, 56, 355.
-
Reaction Mechanism
DMF and phosphorus oxychloride react to form the Vilsmeier reagent, which undergoes SEAr reaction. Hydrolysis of the iminium intermediate gives the formylated product.
-
Examples
A reaction example.[1]
An example employing DMF-Tf2O.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1]
[2] Martinez, A. G.; Alvarez, R. M.; Barcina, J. O.; Cereo, S. M.; Vilar, E. T.; Fraile, A. G.; Hanack, M.; Subramanian, L. R. J. Chem. Soc., Chem. Commun. 1990, 1571.
-
Related Books

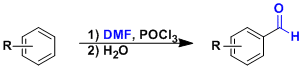
![ar-al-8[1]](https://assets.en.chem-station.com/uploads/2014/04/ar-al-81.gif)
![ar-al-9[1]](https://assets.en.chem-station.com/uploads/2014/04/ar-al-91.gif)
![vilsmeier_4[1]](https://assets.en.chem-station.com/uploads/2014/04/vilsmeier_41.gif)