Teruaki Mukaiyama (born 1927) is an organic chemist in Japan.
He was born on January 5, 1927 in Nagano, Japan. He received his BSc from the Tokyo Institute of Technology (TIT) in 1948 and his PhD from the University of Tokyo in 1957. He became an Assistant Professor at Gakushuin University in 1953 and moved to TIT in 1958. He was appointed Full Professor at TIT in 1963, and then in 1973 moved to the University of Tokyo. After he completed his term at the University of Tokyo in 1987, he moved to the Science University of Tokyo. He became President of the Research Institute for Science and Technology of the same university in 1991, and was given the title of Distinguished Professor in 1992. In 2002, he moved to the Kitasato Institute and worked in there until 2009.
- Education and Experience
1948 BSc from the Tokyo Institute of Technology (TIT)
1953 Assistant Professor at Gakushuin University
1957 Associate Professor at Gakushuin University
1957 Ph. D University of Tokyo
1958 Associate Professor at the Tokyo Institute of Technology (TIT)
1963 Professor at the Tokyo Institute of Technology (TIT)
1973 Professor at University of Tokyo
1987 Professor at Tokyo University of Science
1991 Director of the Research Institute for Science and Technology at Tokyo University of Science
1992 Distinguished Professor, Tokyo University of Science
2002 Emeritus Director of Kitasato Institute
2009 Retirement
- Awards and Honors
1973 Chemical Society of Japan Award
1976 Honorary Degree from Technische UniversitFt MGnchen
1983 Imperial Prize, Japan
1983 Academy Prize, Japan
1985 Honorary Professor of Jilin University, China
1986 Copernicus Medal, Poland
1992 Person of Cultural Merit, Japan
1994 Special Award, Society of Synthetic Organic chemistry, Japan
1994 Chevalier de l:Ordre National du MHrite, France
1996 ACS Award for Creative Work in Synthetic Organic Chemistry
1997 Order of Culture, Japan
1998 Tetrahedron Prize, UK
2006 Sir Derek Barton Gold Medal, UK
- Research
His research interests have covered organic chemistry, organic synthesis, and synthetic methodology including various important topics such as thermal decomposition of ureas and urethanes, organic dehydration, organic deoxygenation, ring-opening polymerization, oxidation–reduction condensation, new synthetic reactions based on organosulfur compounds, titanium and boron compounds, the onium salts of azaaromatics, synthetic control, asymmetric synthesis, carbohydrate synthesis, epoxidation of olefins with molecular oxygen, chiral Lewis acid mediated asymmetric aldol reactions, new oxidation methods, and Lewis base catalysis. He also completed the total synthesis of taxol.
His seminal work on the aldol reaction and related methodology would be among his most eminent contributions to organic chemistry, judging from the importance in the area and the influence on other researchers and fields.[1] The importance of the famous Mukaiyama aldol reaction of silyl enol ethers with aldehydes, ketones, acetals, etc. promoted by TiCl4 cannot be underestimated, and numerous related reactions have also been reported. Prior to Professor Mukaiyama:s contributions to this field, aldol reactions were performed under basic conditions, which induced several undesired side reactions and led to low yields. Professor Mukaiyama challenged this dogma and demonstrated that aldol reactions could be performed under acidic conditions. The Mukaiyama aldol reaction was first extended to catalytic versions and then to catalytic asymmetric versions, and many research groups have actively pursued applications of this methodology.
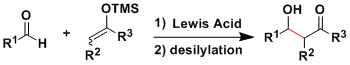
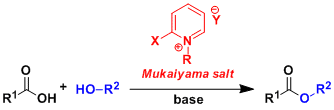
Professor Mukaiyama has also investigated dehydration reactions, which are very important and fundamental reactions in organic synthesis. He first found that intramolecular dehydration reactions of nitroalkanes with isocyanate provided alkyl nitrile oxides, an archetypal 1,3-dipole motif. Through these studies on new dehydrating reagents, he noticed an interesting characteristic of trivalent phosphorus compounds, which led him to develop a new dehydration reaction based on an oxidation–reduction principle to remove H2O as 2[H] and [O]. For example, an ester or amide is prepared from an equimolar amount of a carboxylic acid and an alcohol or amide by combining Ph3P and disulfide under neutral conditions. He also designed onium salts of azaaromatic compounds such as 2-chloro-1-methylpyridinium salts, and various intra- and intermolecular dehydration reactions were carried out in high yields under neutral conditions.
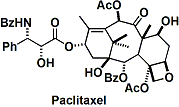
Remarkably, He succeeded in the asymmetric total synthesis of taxol as his first challenge in the total synthesis of natural products.[3] The synthesis was established according to a unique strategy to construct the taxol skeleton in the order B (eight-membered ring) to C to A rings. The B ring was synthesized by using asymmetric and stereoselective aldol reactions that he had developed. In the synthesis, he also employed a new high-yielding method to introduce N-benzoylphenylisoserine to baccatin III by using a dehydration–condensation reaction.
- Photo Gallery
- The 40th Anniversary Symposium of the Mukaiyama Aldol Reaction, Tokyo, Aug. 31, 2013
- The 40th Anniversary Symposium of the Mukaiyama Aldol Reaction, Tokyo, Aug. 31, 2013
- The 40th Anniversary Symposium of the Mukaiyama Aldol Reaction, Tokyo, Aug. 31, 2013
- The 40th Anniversary Symposium of the Mukaiyama Aldol Reaction, Tokyo, Aug. 31, 2013
- Representative Papers
[1] (a) Mukaiyama, T.; Narasaka, K.; Banno, K. Chem. Lett. 1973, 1011. doi:10.1246/cl.1973.1011 (b) Mukaiyama, T.; Izawa, T.; Saigo, K. Chem. Lett. 1974, 323. doi:10.1246/cl.1974.323 (c) Mukaiyama, T.; Banno, K.; Narasaka, K. J. Am. Chem. Soc. 1974,96, 7503. DOI: 10.1021/ja00831a019
[2] Narasaka, K.; Maruyama, K.; Mukaiyama, T. Chem. Lett. 1978, 885. doi:10.1246/cl.1978.885
[3] (a) Shiina, I.; Iwadare, H.; Hasegawa, M.; Tani, Y.; Mukaiyama, T. Chem. Lett. 1998, 1. doi:10.1246/cl.1998.1 (b) Mukaiyama, T.; Shiina, I.; Iwardare, H.; Saitoh, M.; Nishimura, T.; Ohkawa, N.; Sakoh, H.; Nishimura, K.; Tani, Y.; Hasegawa, M.; Yamada, K.; Saitoh, K. Chem. Eur. J. 1999, 5, 121. [abstract] (c) Mukaiyama Taxol Synthesis – Wikipedia (d) 向山光昭、TCIメール 1998, 10, 3 [PDF]
[4] (a) Mukaiyama, T.; Matsuo, J.-i.; Yanagisawa, M. Chem. Lett. 2000, 1072, doi:10.1246/cl.2000.1072 (b) Mukaiyama, T.; Matsuo, J.-i.; Kitagawa, H. Chem. Lett. 2000, 1250. doi:10.1246/cl.2000.1250
[5] (a) Mukaiyama, T.; Shintou, T.; Fukumoto, K. J. Am. Chem. Soc. 2003, 125, 10538. DOI:10.1021/ja0303844 (b)Shintou, T.; Mukaiyama, T.; J. Am. Chem. Soc. 2004, 126, 7359. DOI:10.1021/ja0487877
[6] Isayama, S.; Mukaiyama, T. Chem. Lett. 1989, 1071. doi:10.1246/cl.1989.1071
[7] Review of Mukaiyama’s work: (a) Mukaiyama, T. Tetrahedron 1999, 55, 8609. doi:10.1016/S0040-4020(99)00437-8 (b) Mukaiyama, T. Angew. Chem. Int. Ed. 2004, 43, 5590. doi:10.1002/anie.200300641
[8] Special Issue In Honor of Professor Teruaki Mukaiyama for His 80th Birthday: Chem. Asian J. 2008, vol 3 [link]
- Related Books
[amazonjs asin=”3527332057″ locale=”US” title=”Modern Methods in Stereoselective Aldol Reactions”][amazonjs asin=”1402087004″ locale=”US” title=”Aldol Reactions”]









