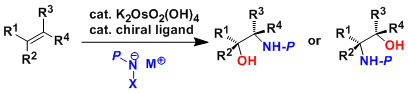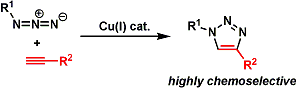Karl Barry Sharpless (born in 1941) is an American chemist who was awarded half the 2001 Nobel Prize in Chemistry for his research into chirally catalysed oxidation reactions. He has discovered and developed many widely used catalytic oxidation processes, including the first general methods for stereoselective oxidation the Sharpless reactions for asymmetric epoxidation, dihydroxylation, and aminohydroxylation of olefins. He also initiated the idea of “click chemistry” where substances are quickly generated from joining small units together.
-
Education and Experiences
1963 BA, Dartmouth College (T. A. Spencer)
1968 PhD, Stanford University (E. E. van Tamelen)
1968 Postdoctoral, Stanford University (J. P. Collman)
1969 postdoctoral, Harvard University (K. Bloch)
1970 Assistant Professor, associate professor, Massachusetts Institute of Technology (MIT)
1977 Professor, Stanford University
1980 Professor, MIT
1987 Arthur C. Cope Professor, MIT
1990 Professor, The Scripps Research Institute
1996 Skaggs Institute for Chemical Biology of TSRI
-
Research
K. Barry Sharpless was born in Philadelphia in 1941. He studied chemistry at Dartmouth College where he did undergraduate research with Thomas A. Spencer to whom he is attached by friendship and a lang collaboration in the field of steroid demethylation (first joint paper in 1964, last in 1975) For his Ph. D. work Sharpless joined E. E. von Tamelen’s group at Stanford (thesis in 1968. “Featuring Enzymic Cyclizatioil of Modified Squalene Oxides”).
In a subsequent postdoctoral period he vacillated between biochemistry (with K. Bloch, Harvard) and inorganic chemistry (with J. Collman, Stanford) until he joined the faculty of the chemistry department at MIT in 1970 and settled for organic chemistry, more specifically, for the oxidation of organic compounds using inorganic reagents.
Among the oxidants he has studied are derivatives of sulfur, seIenium, titanium, vanadium, chromium, molybdenum, tungsten, manganese, ruthenium emd osmium, the corresponding synthetic transformation being hydroxylation, epoxidation and amination of C,C-double bonds (olefin) as weil as allylic amination and oxidation.
The processes for the selective oxidation of olefins have long been among the most useful tools for day-to-day organic synthesis because of these appealing characteristics of olefins:
1) they are among the cheapest functionalized organic starting materials
2) they can be carried ™hidden∫ through conventional acid/base-catalyzed transformations, then ™revealed” at will by adding heteroatoms through selective oxidations
3) most simple olefins are prochiral, and provide a prominent portal to the chiral world.
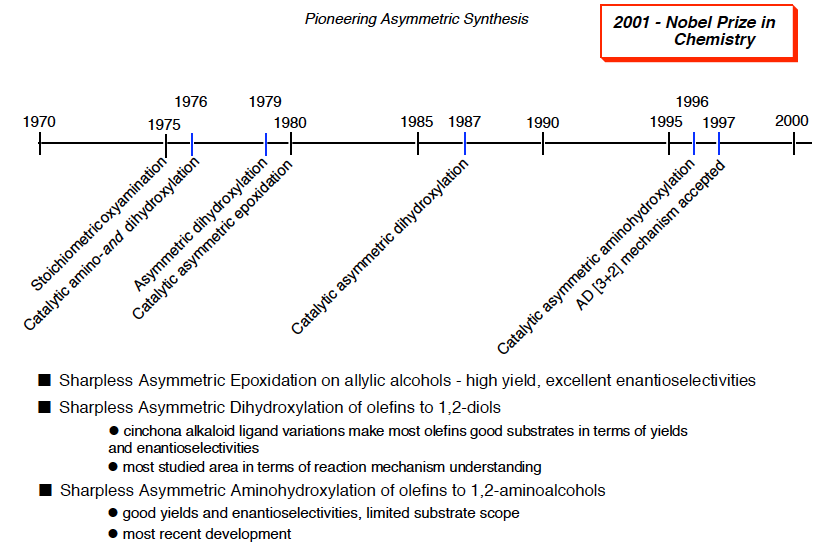
The transition-metal mediated regio- and diastereoselective epoxidation of olefins with t-butyl hydroperoxide(TBHP) was first described by Sharpless and his group in 1973.[1] It took seven more years until the enantioselective version of that reaction was discovered with Katsuki. The stoichiometric or catalytic epoxidation of allylic alcohols by peroxides in the presence of Ti(OiPr)4 and dlethyl tartrate became an instant name reaction: Sharpless-Katsuki asymmetric epoxidation.[2]
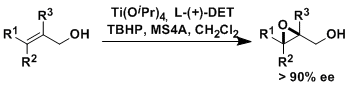
Sharpless–Katsuki asymmetric epoxidation
In same year, an enantioselective catalytic hydroxylation of double bonds with N-oxide/Os04/ chiral base has been developed in Sharpless’s laboratory.[3] During more than 8 years, They improved enantioselectivity and finally found out the optimal conditions (Sharpless asymmetric dihydroxylation).[4] That is performed with an osmium catalyst and a stoichiometric oxidant [e.g. K3Fe(CN)6 or N-methylmorpholine oxide (NMO)]; it is carried out in a buffered solution to ensure a stable pH, since the reaction proceeds more rapidly under slightly basic conditions. Enantioselectivity is achieved through the addition of enantiomerically-enriched chiral ligands [(DHQD)2PHAL, (DHQ)2PHAL or their derivatives]. These reagents are also available as stable, prepackaged mixtures (AD-mix α and AD-mix β, AD = asymmetric dihydroxylation) for either enantiopreference.
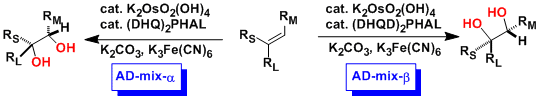
Sharpless asymmetric dihydroxylation
In 1996, Sharpless also discovered asymmetric aminohydroxylation, a route to optically active vicinal amino-alcohols.[5] Cinchona alkaloid ligands make the first asymmetric and catalytic hydroxyamination. (Sharpless asymmetric aminohydroxylation)
Sharpless asymmetric aminohydroxylation
Chronological table of development of asymmetric oxidation by Sharpless is shown below. Sharpless’s methods allow for the manufacture of safer and more effective antibiotics, anti-inflammatory drugs, heart medicines, and agricultural chemicals. Sharpless contributed innovations to the development of broadly useful and commercially viable catalytic oxidation chemistry for the selective production of bioactive chiral molecules with the proper right or left “handedness.” In 2001, Sharpless was awarded Nobel prize in chemistry along with William S. Knowles, formerly of Monsanto, and Ryoji Noyori of Nagoya University in Japan for “the development of catalytic asymmetric synthesis.”
(Ref. https://www.princeton.edu/chemistry/macmillan/group-meetings/nikki-sharpless.pdf)
Sharpless currently focuses on advancing and promoting ‘Click Chemistry.‘[6]. Click chemistry refers to a group of reactions that are fast, simple to use, easy to purify, versatile, regiospecific, and give high product yields. While there are a number of reactions that fulfill the criteria, the Huisgen 1,3-dipolar cycloaddition of azides and terminal alkynes has emerged as the frontrunner. It has found applications in a wide variety of research areas, including materials sciences, polymer chemistry, and pharmaceutical sciences.
Huisgen Cycloaddition
-
References
[1] Sharpless, K. B.; Michaelson, R. C. J. Am. Chem. Soc. 1973, 95, 6136. DOI: 10.1021/ja00799a061
[2] Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 5974. DOI: 10.1021/ja00538a077
[3] Hentges, S. G.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 4263. DOI: 10.1021/ja00532a050
[4] Jacobsen, E. N.; Marko, I.; Mungall, W. S.; Schroder, G.; Sharpless, K. B. J. Am. Chem. Soc. 1988,110, 1968. DOI: 10.1021/ja00214a053
[5] Li, G.; Chang, H.-T.; Sharpless, K. B. Angew. Chem. Int. Ed. Engl. 1996, 35, 451. DOI:10.1002/anie.199604511
・ Nobel Lecture: Sharpless, K. B. Angew. Chem. Int. Ed. 2001, 40, 2024. [DOI]
・ Review: Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem. Int. Ed. 2001, 40, 2004. [DOI]
[6] (a) Sharpless, K. B. et al. Angew. Chem. Int. Ed. 2002, 41, 1053. [DOI] (b) Sharpless, K. B. et al. J. Am. Chem. Soc. 2005, 127, 6686. DOI:10.1021/ja043031t
-
Related Links
The Sharpless LAB
Karl Barry Sharpless - Wikipedia
-
Photo Gallery
-
Movies
-
Related Books
[amazonjs asin=”111807856X” locale=”US” title=”Efficient Preparations of Fluorine Compounds”][amazonjs asin=”3527323201″ locale=”US” title=”Modern Oxidation Methods”][amazonjs asin=”0470699701″ locale=”US” title=”Click Chemistry for Biotechnology and Materials Science”]