- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
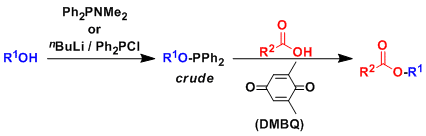
The quinone-phosphine redox system can be used to activate alcohols towards esterification, etherification, and other SN2 reactions with various nucleophiles. The reaction is similar to the Mitsunobu reaction, so the reaction proceeds with the inversion of stereochemistry at the reacting carbon.
Remarkably, even some tertiary alcohols, which under normal circumstances do not undergo SN2 displacement, give inversion products under these conditions.
-
General References
・ Mukaiyama, T.; Shintou, T.; Fukumoto, K. J. Am. Chem. Soc. 2003, 125, 10538. DOI: 10.1021/ja0303844
・ Shintou, T.; Mukaiyama, T.; J. Am. Chem. Soc. 2004, 126, 7359. DOI: 10.1021/ja0487877
・ Recent Review on Mukaiyama’s Work: Angew. Chem. Int. Ed. 2004, 43, 5590. doi:10.1002/anie.200300641
-
Reaction Mechanism
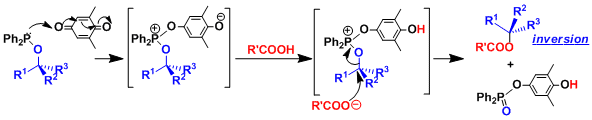
-
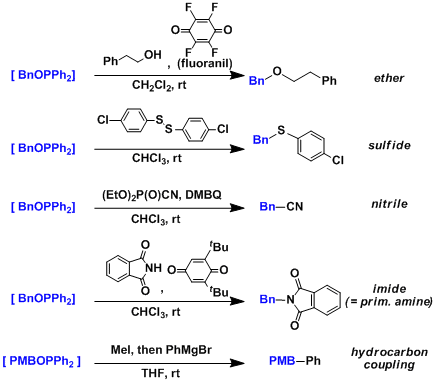
Examples
A wider range of nucleophiles have been shown to be viable reactants compared with the Mitsunobu reaction, for which the nucleophiles need to be reasonably acidic.
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books