- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The oxidation of alcohols using the combination of DCC+Brønsted acid+DMSO is known as the Pfitzner-Moffatt oxidation.
The active species of this reaction is bulkier than those of the Swern and the Parikh-Doering reactions. Taking advantage of the fact that this reaction tends to be sensitive to steric influences, it is possible to oxidize less hindered hydroxyl groups selectively. That this reaction can be carried out at room temperature is another important merit.
The reaction has some disadvantages including the removal of the urea byproduct and the competitive methylthiomethyl ether byproduct formation.
-
General References
・ Pfitzner, K. E.; Moffatt, J. G. J. Am. Chem. Soc. 1963, 85, 3027. DOI: 10.1021/ja00902a035
・ Pfitzner, K. E.; Moffatt, J. G. J. Am. Chem. Soc. 1965, 87, 5661. DOI: 10.1021/ja00952a026
・ Pfitzner, K. E.; Moffatt, J. G. J. Am. Chem. Soc. 1965, 87, 5670. DOI: 10.1021/ja00952a027
・ Review: Tidwell, T. T. Org. React. 1990, 39, 297.
・ Review: Tidwell, T. T. Synthesis 1990, 857. DOI: 10.1055/s-1990-27036
・ Review: Lee, T. V. Comprehensive Organic Synthesis 1991, 7, 291.
-
Reaction Mechanism
The basic mechanistic rationale is similar to that of the Swern oxidation.
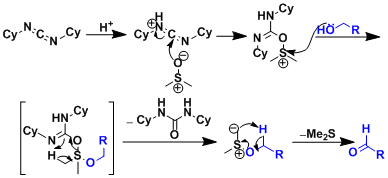
-
Examples
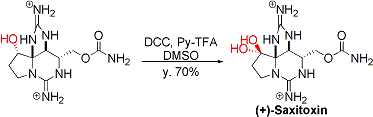
The synthesis of (+)-saxitoxin.[1]
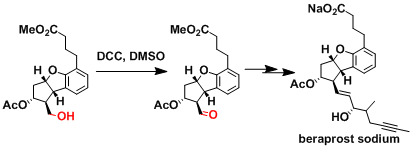
The synthesis of beraprost.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Fleming, J. J.; Du Bois, J. J. Am. Chem. Soc. 2006, 128, 3926. DOI: 10.1021/ja0608545
[2] J. R. Prous ed. Drugs Fut., 1986, 11, 956.
-
Related Books

