Szymanski, W.; Velema, W. A.; Feringa, B. L. Angew. Chem. Int. Ed. 53, 2014. Early View.
DOI: 10.1002/anie.201402665
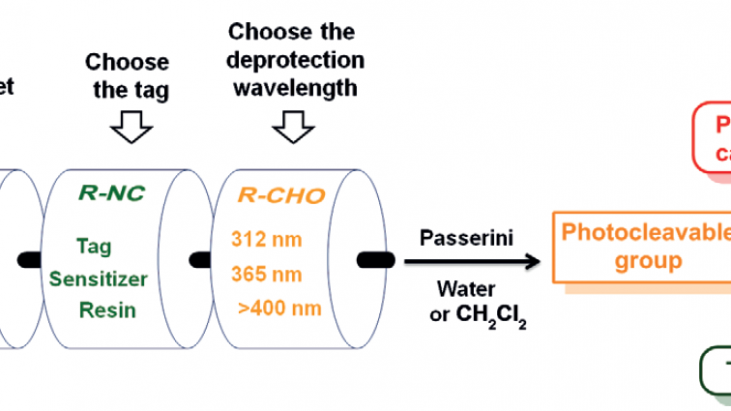
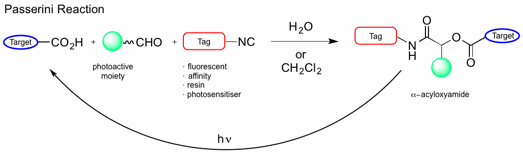
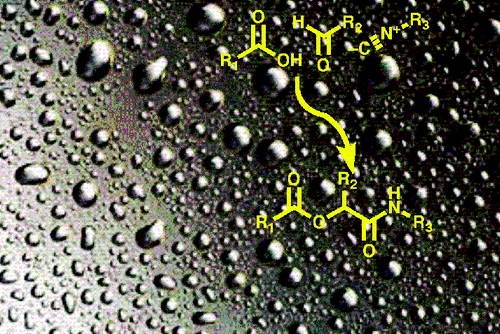
Photocaged compounds are important tools for studying and regulating multiple processes, including biological functions. Reported herein is the use of the Passerini multicomponent reaction for modular preparation of photocaged carboxylic acids. The reaction is compatible with several functionalities and proceeds smoothly both in water and dichloromethane. The choice of aldehyde determines the wavelength used for deprotection and enables formation of orthogonally protected products. The isocyanide component can be used for introduction of reactive tags and photosensitizers, as well as for immobilization on a solid support.
Introducing photolabile protecting groups into bioactive molecules, a process called photocaging, enables molecules to be activated by light. Photocaged molecules are considered to be important tools for studying and controlling biological processes, due to the advantages of using light as a trigger. Light has low toxicity towards cells, tunable intensity and wavelength, along with temporal and spatial control of its delivery. Photocaging of carboxylic acids through attachment of photocleavable groups has commonly been used in many biological processes, including the study of developmental defects in zebrafish embryos, photocontrolled drug delivery and release of agrochemicals. In view of this high utility, Feringa and coworkers reported the first example of introducing photolabile protecting groups to carboxylic acids in aqueous media using the Passerini reaction.
The Passerini reaction, discovered by Mario Passerini in 1921,[1] is a three-component reaction involving an isocyanide, an aldehyde and a carboxylic acid to form an α-acyloxyamide, and has also been successful in water.[2] This reaction has numerous applications, such as for the preparation of dendrimer-drug conjugates, polymers and heterocycles. In this report, Feringa’s group has tuned the substrates for the modular synthesis of photocaged carboxylic acids in both aqueous and organic media. Various aldehydes were explored to enable deprotection of carboxylic acids at different wavelengths. On the other hand, the isocyanate component was modified to introduce various tags, which can be used for visualization (fluorescence), affinity purifications, immobilisation on a solid support or sensitised photodeprotection by intramolecular, photoinduced energy transfer.
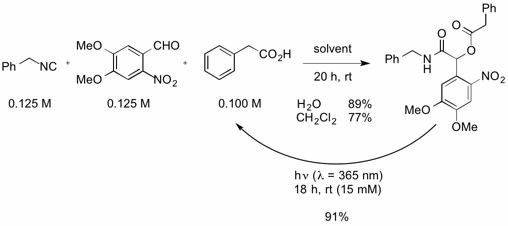
Upon screening various carboxylic acids, it was found that hydrophobic carboxylic acids resulted in high yields for the Passerini reaction. Selective uncaging was observed by deprotection of the adduct under λ = 365 irradiation. The desired acid was released in 91% yield after 18 h of irradiation. Wavelength-selective, orthogonal uncaging of photoprotected bioactive compounds has received considerable attention due to its potential to selectively trigger various responses in biological systems.
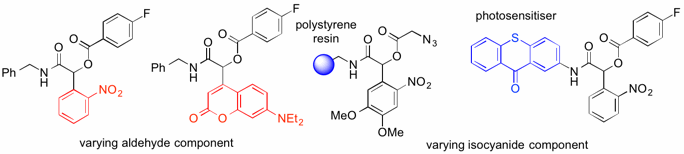
The wavelength used for deprotection depends on the structure of the aldehyde. Different aldehydes were applied to obtain photoprotected products, which showed different spectral properties and could be uncaged by different wavelengths. In order to increase the applicability of this system, the isocyanide component was tuned by attaching various tags to be used for purification, immobilisation on a solid support for solid-phase peptide synthesis, photosensitising, and further functionalisation.
The multicomponent Passerini reaction has shown to be applicable to synthesise photocaged carboxylic acids in both aqueous and organic media. Through tuning of the aldehyde and the isocyanide component, the wavelength for deprotection can be varied and a number of useful tags can be introduced, respectively. This method has been used for photocleavable immobilisation on a solid support, as well as installation of a photosensitiser, thus demonstrating the potential of this reaction to be used for solid-phase synthesis and to prepare compounds with different photochemical properties.
-
References
[1] Passerini, M. Gazz. Chim. Ital. 1921, 51, 181.
[2] “Multicomponent Reactions Are Accelerated in Water”
Pirrung, M. C.; Sarma, K. D. J. Am. Chem. Soc. 2004, 126, 444. DOI: 10.1021/ja038583a
Two multicomponent reactions, the Ugi and Passerini reactions, are accelerated by the use of aqueous solutions. The rate enhancements compared to those by organic solvents can approach 300-fold. Reactions performed in water offer another advantage that products are often insoluble, permitting direct isolation by precipitation. The methods were applied to the preparation of three small combinatorial libraries.
-
Related Books
[amazonjs asin=”3527308067″ locale=”US” title=”Multicomponent Reactions”][amazonjs asin=”3131668814″ locale=”US” title=”Multicomponent Reactions 1 (General Discussion and Reactions Involving a Carbonyl Compound As Electrophilic)”]
-
Related Links
The Feringa Group