In January 2015, Yves Chauvin, the French chemist who was the first to propose the reaction mechanism of olefin metathesis, passed away. For his contribution, he shared the Nobel Prize in Chemistry in 2005 with Robert Grubbs and Richard Schrock, who developed first well-defined catalysts.
As you all know, olefin metathesis (or olefin cross-metathesis) is the reaction in which the double bonds of two different olefins can be swapped to produce a new pair of olefins. The advent of this extraordinary reaction was a major game-changer in organic synthesis, and it didn’t take long until the reaction became one of the essential tools of synthetic chemists.
Important modifications have been made to the original catalysts and substrate generality has improved considerably for E-selective olefin metathesis. For example, the ruthenium-based “second generation Grubbs-Hoveyda catalyst” is known as a particularly effective catalyst (shown in the figure below).
On the other hand, however, Z-selective metathesis is fundamentally more challenging and the substrate scope has remained much more limited.
In this context, a new example of Z-selective cross-metathesis using novel ruthenium catalysts was reported in the end of last year by the group of Amir Hoveyda of Boston College, who is one of the leaders in this field.
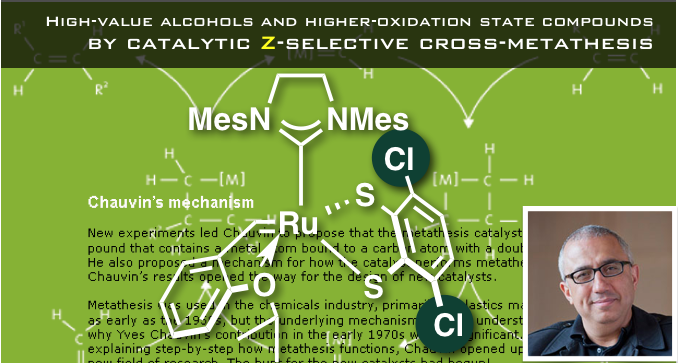
“High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis”
Koh, M. J.; Khan, R. K. M.; Torker, S.; Yu, M.; Mikus, M. S.; Hoveyda, A. H. Nature 2015, 517, 181. DOI:10.1038/nature14061
They were successful in obtaining olefins with high Z-selectivity for substrates containing alcohol and carboxylic acid groups, many of which had previously been either difficult or completely unviable.
So what do the catalysts look like and how were they able to solve the long-standing problem? Let us take a look.
Z-Selective Cross Metathesis
Z-Selective cross metathesis had been very difficult as mentioned, but it was finally reported by the groups of Hoveyda and Schrock in 2011.[1] This chemistry was introduced on this website before so we’ll only touch it briefly this time. As shown below, the reaction effectively produced Z-olefins from enol ethers and allylic amides by employing the molybdenum alkylidene complex.
Reports of Z-selective cross-metathesis by several other groups followed it, but few of those systems were general enough to be compatible with substrates containing reactive functional groups of the likes of aldehyde and carboxylic acid. Towards solving this problem, the researchers had to reexamine the central metal atom of the catalyst.
Development of the New Ruthenium Catalysts
In general, molybdenum and tungsten catalysts are deactivated in the presence of unprotected oxygen-containing functional groups such as alcohol, aldehyde, and carboxylic acid. In contrast, ruthenium dichloride complexes are known to catalyze metathesis unaffected by these groups.
The authors first looked at the two ruthenium complexes (Ru-2 and Ru-3a), which contain anionic ligands similar to the dichloride complexes.
These complexes had been demonstrated to be effective catalysts for Z-selective homo-metathesis and ring-opening metathesis reactions.[2] Indeed, these catalysts worked for cross-metathesis of alcohol-containing substrates, and the authors ultimately found that Ru-3b, the derivative of Ru-3a, was the most active and selective.
The improved catalytic activity of Ru-3b was attributed to the suppression of catalyst decomposition thanks to the introduction of chlorine atoms. For those of you who are interested in learning more about the details, we would recommend checking out the paper that explains it with DFT energy calculation.
The newly developed catalyst was demonstrated to be effective not only for substrates containing alcohol, aldehyde, and carboxylic acid, but also for some sterically hindered substrates and diene substrates.
The authors also applied it to the stereoselective synthesis of the substructure of natural products such as neopeltolide and leucascandrolide A. They went on to complete the total synthesis of (+)-neopeltolide.[3]
In yet another application, the new catalytic system was successfully applied to the direct conversion of oleyl alcohol and oleic acid, both of which are abundant raw materials found in animal fats and vegetable oils, into high-value Z-alkene products.
The system is different from previously known systems in that: 1) It does not require protecting group manipulation, and 2) The reaction proceeds at lower temperature, which leads to higher Z-selectivity. The high stereoselectivity also helps simplify otherwise-difficult separation of olefin isomers. Please check the paper for more detailed information.
Well, what did you think? As said in the title, it may not be an exaggeration to say that olefin metathesis has now overcome nearly every functional group.
With the expanding substrate scope, the reaction will continue to contribute to the development of organic synthesis. We are saddened by the loss of a great chemist who pioneered this field, but his legacy will always be remembered.
References
- Meek, S. J.; O’Brien, R. V.; Llaveria, J.; Schrock, R. R.; Hoveyda, A. H. Nature 2011, 471, 461. DOI:10.1038/nature09957
- Khan, R. K. M.; Torker, S.; Hoveyda, A. H. J. Am. Chem. Soc. 2013, 135, 10258. DOI: 10.1021/ja404208a
- Yu, M.; Schrock, R. R.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2015, 54, 215. DOI:10.1002/anie.201409120
Books
[amazonjs asin=”3527334246″ locale=”US” title=”Handbook of Metathesis, 3 Volume Set”]
External Links
- Hoveyda Research Group: The group at Boston College that developed the present system.

![5f19f8038b959234c30f03075d8068f41[1]](https://assets.en.chem-station.com/uploads/2015/02/5f19f8038b959234c30f03075d8068f411.jpg)



