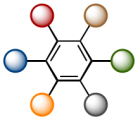
2015 marks the 150th anniversaries of seminal discovery by Kekulé that benzene nucleus are cyclic. [1] This year is also 190th since the first report of benzene by Faraday in 1825. Benzene represents the concepts of aromaticity and delocalization that have been of paramount significance in a variety of fields in chemistry. In addition, due to the high stability as well as the unique properties, benzene has been widely utilized as a structural unit in industrial products such as dyes, plastics, electronics materials, and even pharmaceuticals. The various natures induced from benzene highly depend on the substituents. In other words, if their synthesis can be done at will, we would control the chemical and physical behaviour of benzene derivatives. Among them, hexaarylbenzenes which features six different aryl groups over the C6 skeleton of benzene have been recognized as highly challenging synthetic targets because of the limitation in constructing such starburst motifs. Hence, a truly general mean is prerequisite for that.
So, what kinds of approaches are plausible to introduce six aryl groups into benzene skeleton at will? One might propose successive cross-coupling reactions employing the different aryl groups. However, it seems not practical as the problem on the control of the position of substitution could occur. Further, the last coupling reaction would be hampered by the steric repulsion. Then, what about the [2+2+2] cycloaddition using diarylacetylene? This method also seems to involve a difficulty in controlling the position of substituents.
Here I would like to introduce a recent “Cool” paper demonstrating a beautiful chemistry on the synthesis of hexaarylbenzene.
“Synthesis and characterization of hexaarylbenzenes with five or six different substituents enabled by programmed synthesis”
Suzuki, S.; Segawa, Y.; Itami, K.; Yamaguchi. J. Nat. Chem. 7, 2015. 26-Jan online. (DOI: 10.1038/nchem.2174)
In this paper, Yamaguchi, Itami, and co-workers report a successful approach which is based on Diels-Alder reaction between thiophen derivatives and alkynes.
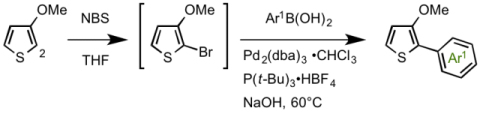
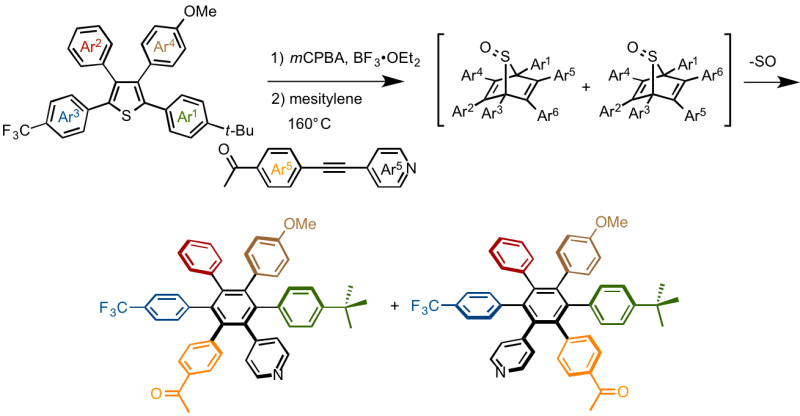
Synthesis of thiphene derivatives bearing four different substituents
Yamaguchi, Itami and coworkers developed a method to prepare thiophene and thiazole derivatives bearing four or three different substituents in advance. [2]
3-methoxy-thiophene was selected as the starting material because the methoxy group acts as a directing group at the initial step, and can be functionalized in the later reaction. Although they had invented a method to introduce the first aryl group Ar1 at 2-position of the thiophene directly via a C–H coupling reaction, to increase the yields, here they employed one-pot two-step reaction involving bromination and Suzuki-Miyaura cross-coupling.
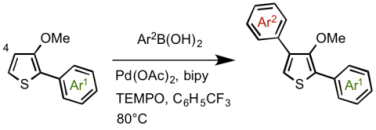
2nd aryl group Ar2 was introduced at 4-position via a direct C-H coupling reaction in which they improved the extant reaction condition to increase the yields.
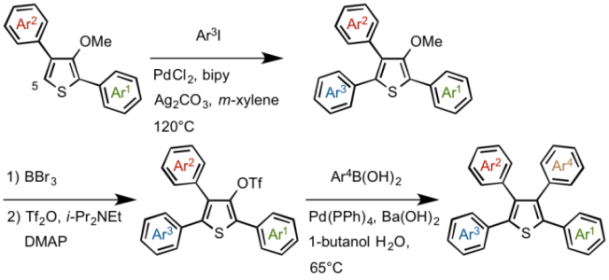
After the introduction of 3rd aryl group Ar3 at 5-position via a C-H coupling reaction, the methoxy group was converted to OTf group, and then 4th aryl group Ar4 was attached at 3-position by Suzuki-Miyaura cross-coupling reaction, completing the programmed synthesis of thiophene derivatives featuring four different aryl groups Ar1 Ar2 Ar3 Ar4.
Road to hexaarylbenzenes
In advance of [2+4] cycloaddition reaction, thiophene derivatives were oxidized with m-CPBA in the presence of BF3·OEt2 to afford the corresponding sulfoxides. These thiophene S-oxides were mixed with asymmetrical diarylethynes, and [2+4] cycloaddition (Diels-Alder reaction) between them generated bicyclo[1.2.2] intermediates from which hexaarylbenzenes (HAB) were obtained via desulfurization.
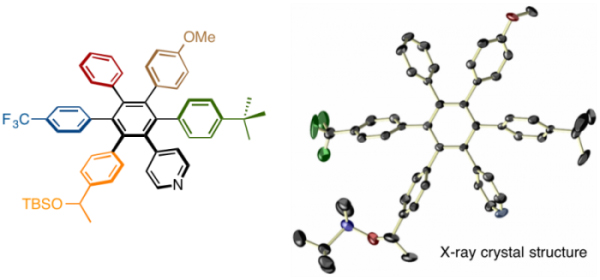
Note that the products involved two regio isomers, and because of that, authors had difficulty on the isolation and the structural characterization. After strenuous efforts in which authors converted the carbonyl group to siloxy group, isolation of one of the isomers was successfully accomplished and X-ray crystallographic analysis revealed the beautiful starburst structure as shown below.
What is programmed synthesis?
Programmed synthesis refers to the synthetic methodology to allow constructing a certain molecular motif bearing several substituents in which myriad combinations of different substituents are, in principle, possible.
Since any kinds of hexaarylbenzenes (HAB) can be prepared using the strategy described herein, this achievement can be considered as the first programmed synthesis for hexaarylbenzene (HAB)! Indeed, this study demonstrated the power and value of synthetic methodology to install a variety of substituents in a predictable manner. The control of regio-selective Diels-Alder, the last step in the set of these reactions would be next challenge.
By the way, according to Burnside’s counting theorem [ref 3], the number of plausible substituted benzenes (N) from n different substituents can be estimated by the following formula: (2n + 2n2 + 4n3 + 3n4 + n6)/12. For instance, with six aryl groups (n = 6), the number of substitution pattern is 4291. If ten aryl groups are available (n = 10), N tremendously increases up to 86,185. Among such countless compounds, molecule exhibiting unusual property must be included.
Authors demonstrated an elegant way to install all different aryl groups into a simple benzene skeleton, which is very important from both fundamental and application points of view.
Authors comments
Chem-Station got comments from authors in this report!
<Story on this challenging project (By Prof. Yamaguchi, 30th– Jan. 2015)>
~Prof. Itami (one of the Principal Investigators in this paper) had dreamed the development of programmed synthesis of hexaarylbenzene (HAB) for a long time. In 2005 when he started the group at Nagoya University, one of the undergrads (year 4) was given the project: “Synthesis of HAB from methoxybenzene through C-H coupling reaction”. Unfortunately, the project did not work well, and they could not but change the direction.
The undergrad went on to graduate course in the same group, and he developed “programmed synthesis of hexaarylthiophene”, instead of HAB.
Prof. Yamaguchi (one of the Principal Investigators in this paper) considered the thiophen as a C4 unit, and reasoned that the addition of a C2 unit such as acetylene to the thiophen would provide a C6 unit, namely, a benzene skeleton. In 2012, Prof. Yamaguchi started to attempt “destroy” hexaarylthiophen so as to produce hexaarylbenzene (HAB) from that. Although Prof. Itami was slightly hesitant on starting this, he assigned an undergrad to the project for Prof. Yamaguchi.
At first they needed to improve the synthetic method for the tetraarylthiophenes because the previous method afforded only a few mg of the products. The undergrad, Mr. Suzuki (actually he is the first author of this paper), developed a method to produce the hexaarylthiophenes in gram scale. Oxidation of thiophenes resulted in the higher reactivity of derivatives as “dienes”, which allowed the [4+2] cyclisation reaction with various symmetric diarylethynes to produce pentaarylbenzenes. Suzuki got the X-ray structure of one of the products on the Christmas day which was wonderful gift to him.
Next authors attempted the reaction of hexaarylthiophenes with asymmetrical diarylethyne. As predicted, however, the reaction product was a mixture of isomers and the control of the regioselectivity of this reaction was not successful. As such, authors nearly gave up, and discussed to complete the project as “Synthesis of hexaarylbenzene with five different substituents”, which was also hitherto unknown at that time. However, Prof. Yamaguchi was still eager to accomplish the synthesis of hexaarylbenzene (HAB), and asked Suzuki to continue the separation of the regioisomers. Suzuki placed on his effort on the isolation and finally found that six times recrystallization affords pure hexaarylbenzene (HAB). Unfortunately, however, the structural characterization was hampered by the problem of disorder in X-ray analysis. Because Suzuki had already received a job offer from company, their time for this project was limited. After all, the products were derivatized to other structures synthetically, and its purification afforded a pure single crystal. At the beginning of summer in 2014, X-ray diffraction analysis successfully revealed the pure structure of haxaarylbenzene. Prof. Yamaguchi still remembers hearing Prof. Itami’s happy screaming sounded in the whole hallway.
During the summer, authors synthesized various derivatives such as pentaarylpyridines, tetraarylnaphthalenes by varying the triple bond fragments, and fully characterized. When the manuscript was completed to submit, Suzuki had been strongly attracted by the chemistry of beautiful hexaarylbenzenes, and in fact he declined the job offer so that he could continue to pursue this chemistry through PhD program.
“There were two reasons why I set this project” Prof. Yamaguchi says. “First, I wanted to synthesize ‘Cool’ molecule such as a cycloparaphenylene developed by Prof. Itami. Second, I wanted to challenge the synthesis of unaccomplished novel molecules, as a synthetic chemist”. Prof. Yamaguchi thanks Mr. Suzuki for his efforts with high synthetic skills to achieve the project.~
This milestone paves the way for the further development of synthetic chemistry.
References
[1a] “It Began with a Daydream: The 150th Anniversary of the Kekulé Benzene Structure”
Rocke, A. J. Angew. Chem. Int. Ed. 2015, 54, 46 – 50. DOI: 10.1002/anie20140834
In January 1865, August Kekulé (see picture) published his theory of the structure of benzene, which he later reported had come to him in a daydream about a snake biting its tail. Although other theories had been postulated before 1865, Kekulé was the first to identify the correct structure. Kekulé’s theory resulted in a clear understanding of aromatic compounds and thus had a major impact on the development of chemical science and industry.
[1b] “A molecule with a ring to it” Francl, M. Nat. Chem. 2015, 7, 6 – 7. DOI: 10.1038/nchem.2136
January 2015 marks the 150th anniversary of chemistry’s most famous waking dream — the publication of August Kekulé’s paper proposing that the hexavalent benzene nucleus was cyclic, a notion that many years later Kekulé claimed came to him in a daydream. Musing on what makes a genius at a gathering in Berlin’s Rathaus in 1890 to celebrate the 25th anniversary of his publication, Kekulé recounted the vision, in which a snaking line of atoms “captured its own tail and whirled mockingly before [his] eyes.”
[2] (a) “Programmed Synthesis of Tetraarylthiophenes through Sequential C-H Arylation”
Yanagisawa, S.; Ueda, K.; Sekizawa, H.; Itami, K. J. Am. Chem. Soc. 2009, 131, 14622-14623. DOI: 10.1021/ja906215b
(b)“Programmed synthesis of arylthiazoles through sequential C–H couplings”
Tani, S.; Uehara, N. T.; Yamaguchi, J.; Itami, K. Chem. Sci. 2015, 5, 123 – 135. (DOI: 10.1039/c3sc52199k)
A programmed synthesis of privileged arylthiazoles via sequential C–H couplings catalyzed by palladium or nickel catalysts has been accomplished. This versatile protocol can supply all possible arylthiazole substitution patterns (2-aryl, 4-aryl, 5-aryl, 2,4-diaryl, 2,5-diaryl, 4,5-diaryl, and 2,4,5-triaryl) from an unfunctionalized thiazole platform by 11 distinct synthetic routes. We have generated over 150 arylthiazoles by using this methodology. We have applied this method to the rapid synthesis of fatostatin (SREBP inhibitor), and the gram-scale synthesis of triarylthiazoles has been demonstrated.
[3a] Theory of Groups of Finite Order (Cambridge Univ. Press, 1897).
Burnside, W.
[3b] Combinatorial Enumerations of Groups, Graphs, and Chemical Compounds (Springer, 1987).
Polya, G.; Reade, R. C.