- Generality
- Reagent Availability
- Experimental User Friendliness
- Safety and Environmental Impact
- Criteria #5
-
General Characteristics
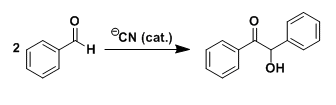
In the presence of nucleophilic catalysts such as cyanide, aromatic aldehydes dimerize to form products called benzoins. Benzoin derivatives are important intermediates for the synthesis of a variety of compounds including deoxybenzoins, benzils, hydrobenzoins, enediols, stilbenes, diphenylethylamines, aminoalcohols, and isoquinolines.
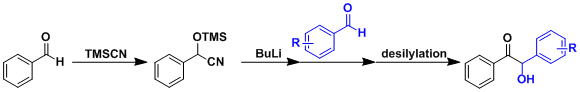
Some benzoins isomerize easily between the keto and the enol forms. Unsymmetrical benzoins equilibrate to the more stable isomer under thermodynamic conditions (the enediol rearrangement). A practical way to synthesize a desired unsymmetrical benzoin is to cyanosilylate one of the aromatic aldehydes first and deprotonate it with a strong base, and then react it with another aromatic aldehyde (as shown below).
Besides cyanide, N-heterocyclic carbenes (NHCs) formed by deprotonation of imidazolium and thiazolium salts are known to act as effective catalysts. Asymmetric benzoin reactions utilizing chiral NHCs have been reported.
-
General References
- Lapworth, A. J. J. Chem. Soc. 1903, 83, 995.
- Lapworth, A. J. J. Chem. Soc. 1904, 85, 1206.
- Staudinger, H. Ber. 1913, 46, 3530, 3535.
- Ide, W. S.; Buck, J. S. Org. React. 1948, 4, 269.
- Hassner, A.; Rai, K. M. L. Comp. Org. Syn. 1991, 1, 541
-
Reaction Mechanism
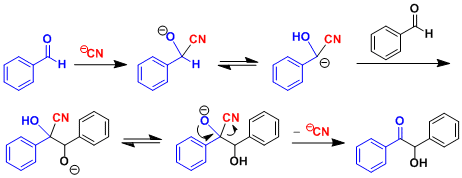
The aldehyde undergoes the reversal of polarity (“umpolung” in German) at its carbonyl carbon upon the addition of the catalyst, after which it reacts as an acyl anion equivalent. For both cyanide and NHCs, the catalyst needs to be capable of stabilizing the anion formed at the α-position.
Catalysis by cyanide
Catalysis by N-Heterocyclic carbene
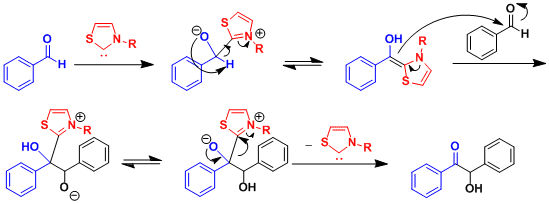
-
Examples
Sila-benzoin reaction[1]: The crossed-benzoin reaction was achieved by clever utilization of acyl silanes and the Brook rearrangement. The chiral metallophosphite catalysts have been used to achieve high levels of enantioselectivity.[1c]
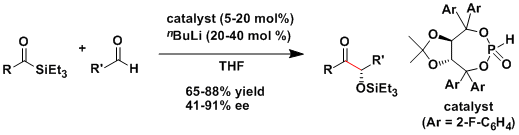
The NHC derived from thiazolium salt can be applied to aliphatic aldehydes.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] (a) Linghu, X.; Johnson, J. S. Angew. Chem. Int. Ed. 2003, 42, 2534. doi:10.1002/anie.200250554 (b) Linghu, S.; Bausch, C. C.; Johnson, J. S. J. Am. Chem. Soc. 2005, 127, 1833. DOI: 10.1021/ja044086y (c) Linghu, X.; Potnick, J. R.; Johnson, J. S. J. Am. Chem. Soc. 2004, 126, 3070. DOI: 10.1021/ja0496468 [2] Stetter, H.; Kuhlmann, H. Org. Synth. 1984, 62, 170.[PDF]
-
Related Books