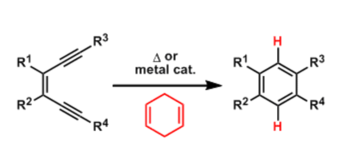
- Generality
- Reagent Availability
- Experimental User Friendliness
- Biochemical Significance
- Criteria #5
-
General Characteristics
When heated at above 200 °C, cis-1,5-hexadiyne-3-ene cyclizes to form 1,4-benzene biradical. The highly reactive biradical species becomes benzene in the presence of hydrogen donors.
The rate of cyclization is known to be dependent on the distance between the bond-forming carbon atoms. The cyclization occurs readily when the distance is closer than 3.34 Å.
-
General References
- Darby, N.; Kim, C.U.; Salaun, J.A.; Shelton, K.W.; Takada, S.; Masamune, S. J. Chem. Soc. 1971, 23, 1516.
- Jones, R. R.; Bergman, R. G. J. Am. Chem. Soc. 1972, 94, 660. DOI: 10.1021/ja00757a071
- Bergman, R. G. Acc. Chem. Res. 1973, 6, 25. DOI: 10.1021/ar50061a004
- Nicolaou, K. C. et al. Proc. Natl. Acad. Sci. USA 1993, 90, 5881.
- Nicolaou, K. C. et al. Chem. Ber. 1994, 41, 33.
- Grissom, J. C. et al. Tetrahedron 1994, 50, 4635. doi:10.1016/S0040-4020(01)85004-3
- Bowles, D. M.; Palmer, G. J.; Landis, C. A.; Scott, J. L.; Anthony, J. E. Tetrahedron 2001, 57, 3753. doi:10.1016/S0040-4020(01)00247-2
- Basak, A.; Mandal, S.; Bag, S. S. Chem. Rev. 2003, 103, 4077. DOI: 10.1021/cr020069k
- Rawat, D. S.; Zaleski, J. M. Synlett 2004, 393. DOI: 10.1055/s-2004-815422
-
Reaction Mechanism
(Ref:J. Org. Chem. 1994, 59, 5833)
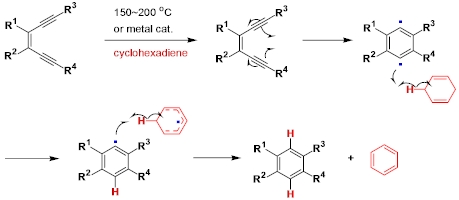
-
Examples
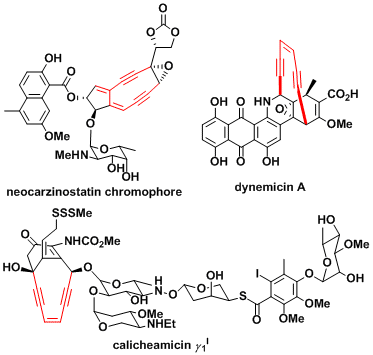
Enediyne natural products such as neocarzinostatin, dynemicin, and calicheamicin contain an enediyne and a DNA-recognition moiety. These compounds show anti-cancer activity because the biradical formed by the Bergman cyclization reacts with the DNA of cancer cells.
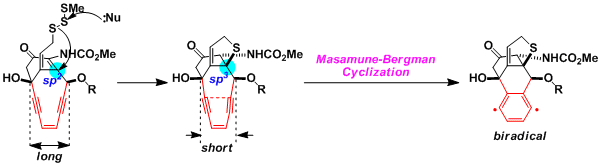
The cyclization of enediyne natural products does not occur spontaneously, but is triggered by biological reactions, which shorten the distance between the termini of the enediyne. The case of calicheamicin is shown here as an example. The nucleophilic attack to the trisulfide triggers the intramolecular 1,4-addition, which leads to the formation of a sp3-hybridized carbon. The change in overall shape of the molecule results in shortened distance between the reacting carbon atoms, promoting the Bergman cyclization.
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books