- Generality
- Orthogonality to Other PGs
- Criteria #3
- Criteria #4
- Criteria #5
-
General Characteristics
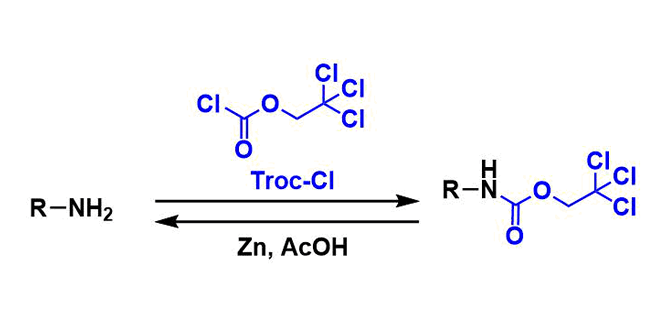
2,2,2-Trichloroethoxycarbonyl (Troc) group is a commonly used carbamate protecting group for amines. It is also used to protect alcohols and phenols.
Troc group is stable under hydrolytic, strongly acidic, nucleophilic, and mild reductive conditions. It can be deprotected under single electron reduction conditions using zinc-acetic acid.
-
General References
- Windholz, T. B.; Johnston, D. B. R. Tetrahedron Lett. 1967, 8, 2555. doi:10.1016/S0040-4039(00)70346-7
- Carson, J. F. Synthesis 1981, 268. doi:10.1055/s-1981-29408
- Just, G.; Grozinger, K. Synthesis 1976, 457. DOI: 10.1055/s-1976-24080
-
Reaction Mechanism
Protection
2,2,2-Trichloroethyl chloroformate (Troc-Cl) is usually used to protect amines. The side products of this reaction are 1,1-dichloroethylene and carbon dioxide.

Deprotection
Using zinc dust, Troc can be deprotected selectively in the presence of other protecting groups. The side products are 1,1-dichloroethylene and carbon dioxide.

-
Examples
An example of selective deprotection of Troc group in the presence of other functional groups.[1]

-
Experimental Procedure
-
References
Bergeron, R. J.; McManis, J. S. J. Org. Chem. 1988, 53, 3108–3111. DOI: 10.1021/jo00248a037
-
Related Reactions
-
Related Books
-
External Links