- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
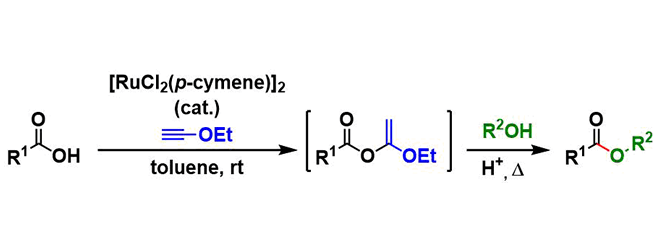
Under the conditions developed by Kita, esters are synthesized from the corresponding alcohols and carboxylic acids using a ruthenium catalyst and ethynyl ethyl ether as a dehydrating agent. The reaction proceeds cleanly under mild conditions, thus workup and purification are made easy.
These conditions have also been used to synthesize acid anhydrides, amides, and macrolactones.
-
General References
- Wasserman, H. H.; Wharton, P. S. J. Am. Chem. Soc. 1960, 82, 661. DOI: 10.1021/ja01488a042
- Kita, Y.; Akai, S.; Ajimura, N.; Yoshigi, M.; Tsugoshi, T.; Yasuda, H.; Tamura, Y. J. Org. Chem. 1986, 51, 4150. doi: 10.1021/jo00372a010
- Kita, Y.; Maeda, H.; Omori, K.; Okuno, T.; Tamura, Y. Synlett 1993, 273. DOI: 10.1055/s-1993-22428
- Kita, Y.; Maeda, H.; Omori, K.; Okuno, T.; Tamura, Y. J. Chem. Soc. Perkin Trans. 1 1993, 2999. doi:10.1039/P19930002999
- Trost, B. M.; Chisholm, J. D. Org. Lett. 2002, 4, 3743. doi: 10.1021/ol026726c
- Ohba, Y.; Takatsuji, M.; Nakahara, K.; Fujioka, H.; Kita, Y. Chem. Eur. J. 2009, 15, 3526. doi: 10.1002/chem.200801548
Reviews
- Kita, Y.; Akai, S. Chem. Rec. 2004, 4, 363. doi:10.1002/tcr.20027
- Parenty, A.; Moreau, X.; Campagne, J.-M. Chem. Rev. 2006, 106, 911. DOI: 10.1021/cr0301402
-
History
The reaction was developed by Professor Yasuyuki Kita and his colleagues in 1986 (then at Osaka University).
-
Reaction Mechanism
Ethynyl ethyl ether is activated by the ruthenium catalyst and reacts with carboxylic acid. The resulting 1-ethoxyvinyl ester is then activated by Brønsted acid and attacked by alcohol, giving ester product and eliminating ethyl acetate.
-
Examples
The total synthesis of amphidinolide A.[1]
An example in optimization of macrolactonization conditions[2]: The Kita conditions afforded the desired product in this case where other commonly used conditions such as the Yamaguchi and the Corey-Nicolaou conditions did not work as well.
-
Experimental Procedure

Macrolactonization[2]
Under nitrogen atmosphere, ethoxyacetylene (21 uL, 0.30 mmol) was slowly added to the solution of [RuCl2(p-cymene)]2 (1.2 mg, 1.99 umol) in acetone (0.5 mL) at 0 ℃. The resulting mixture was stirred at 0 ℃ for 5 min and 13-hexyloxacyclotridecan-2-one (30 mg, 0.098 mmol) in acetone (0.5 mL) was slowly added to the solution at 0 ℃. The resulting mixture was stirred at room temperature for 1 h. Ru was filtered through short neutral SiO2 pad column eluted by EtOAc. The residue was concentrated in vacuo. The ethoxyvinyl ester (EVE) (quant., >95% purity based on 1H NMR analysis) was used without further purification.
The crude EVE was diluted with DCE (10 mL) and slowly added by syringe pump to the highly diluted p-TsOH (0.05 M in DCE/CH3CN 1:1, 0.2 mL, 0.01 mmol) solution in DCE (30 mL) over 10 h at 80 ℃. The reaction mixture was stirred at 80 ℃ for additional 1 h and cooled to room temperature. Et3N (ca. 0.012 mmol) was added and the mixture was concentrated in vacuo. Purification of the residue by SiO2 column chromatography (hexane/Et2O 20:1) gave the desired maclolactone product (18 mg, 64%) as a colorless oil.
-
Experimental Tips
-
References
- (a) Trost, B. M.; Chisholm, J. D.; Wrobleski, S. T.; Jung, M. J. Am. Chem. Soc. 2002, 124, 12420. DOI: 10.1021/ja027883+ (b) Trost, B. M.; Harrington, P. E. J. Am. Chem. Soc. 2004, 126, 5028.DOI: 10.1021/ja049292k
- Ohba, Y.; Takatsuji, M.; Nakahara, K.; Fujioka, H.; Kita, Y. Chem. Eur. J. 2009, 15, 3526. doi: 10.1002/chem.200801548
-
Related Reactions
-
Related Books
-
External Links