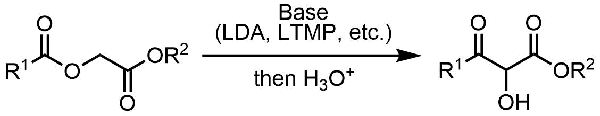Overall Score3.5
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The treatment of α-acyloxyesters with a strong base triggers rearrangement that leads to the formation of α-hydroxy-β-ketoesters.
-
General References
S. D. Lee, T. H. Chan, K. S. Kwon, Tetrahedron Lett., 1984, 25, 3399-3402.
DOI: 10.1016/S0040-4039(01)91030-5
-
Reaction Mechanism

-
Examples
The Chan rearrangement was used in the total synthesis of taxol by Holton et al., where the cyclic carbonate moiety was efficiently converted to γ-lactone moiety.[1]
 The yield of this reaction was moderate in the original report. The examples of the Chan rearrangement utilized in total synthesis are scarce or none other than this, however, Reissig et al. observed it as a side reaction during the synthesis of a model compound of heliquinomycin.[2]
The yield of this reaction was moderate in the original report. The examples of the Chan rearrangement utilized in total synthesis are scarce or none other than this, however, Reissig et al. observed it as a side reaction during the synthesis of a model compound of heliquinomycin.[2]

-
Experimental Procedure
-
Experimental Tips
-
References
- R. A. Holton, C. Somoza, H. B. Kim, F. Liang, R. J. Biediger, P. D. Boatman, M. Shindo, C. C. Smith, S. Kim, H. Nadizadeh, Y. Suzuki, C. Tao, P. Vu, S. Tang, P. Zhang, K. K. Murthi, L. N. Gentile, J. H. Liu, J. Am. Chem. Soc., 1994, 116, 1597-1598.
DOI: 10.1021/ja00083a066 - C. Venkatesh, H.-U. Reissig, Synthesis, 2008, 22, 3605-3614.
DOI: 10.1055/s-0028-1083194
-
Related Reactions
-
Related Books
-
External Links