- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
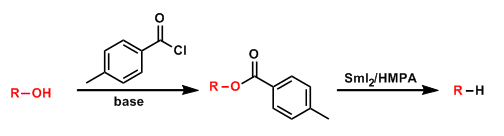
The deoxygenation of alcohols via p-toluic acid esters under single-electron reduction conditions is known as the Marko-Lam deoxygenation.
It has advantages over the Barton-McCombie deoxygenation, including higher stability of the precursors, faster and milder reaction conditions, and unnecessity of toxic (and difficult to remove) organic tin reagents.
-
General References
Lam, K.; Marko I.E. Org. Lett. 2008, 10, 2773. DOI: 10.1021/ol800944p
Lam, K.; Marko I.E. Chem. Comm. 2009, 95. DOI: 10.1039/b813545b
Lam, K.; Marko I.E. Org. Lett. 2009, 11, 2752. DOI: 10.1021/ol900828x
Lam, K.; Marko I.E. Tetrahedron 2009, 65, 10930. doi:10.1016/j.tet.2009.09.111
-
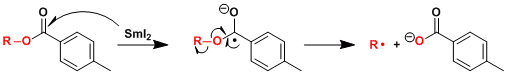
Reaction Mechanism
The resulting alkyl radical intermediate can be subjected to further reactions such as cyclization.
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
Intramolecular Radical Cyclization
Conversion from Alcohol to Alkane
-
Related Books
-
External Links
Marko-Lam Deoxygenation – Wikipedia
Alkane synthesis by deoxygenation (organic-chemistry.org)