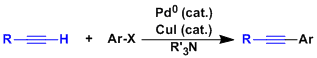- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-The cross coupling reaction between an aryl or alkyl halide and a terminal alkyne catalyzed by the combination of Pd(0), copper iodide, and an amine is called the Sonogashira-Hagiwara reaction. Systems in which the reaction proceeds without palladium or copper are beginning to emerge in recent years.
-
General References
・ Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron lett. 1975, 50, 4467. DOI:10.1016/S0040-4039(00)91094-3
・ Sonogashira, K. Comprehensive Organic Synthesis 1991, 3, 521.
・ Sonogashira, K. J. Organomet. Chem. 2002, 653, 46. DOI:10.1016/S0022-328X(02)01158-0
・ Negishi , E.-i.; Anastasia, L. Chem. Rev. 2003, 103, 1979. DOI: 10.1021/cr020377i
・ Chinchilla, R.; Najera, C. Chem. Rev. 2007, 107, 874. DOI: 10.1021/cr050992x
-
Reaction Mechanism
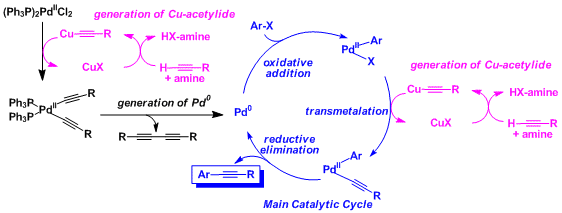
-
Examples
An example in the synthesis of gingkolide.[1]
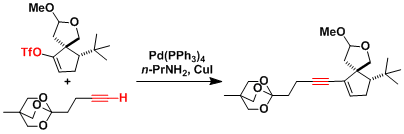
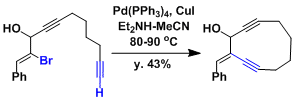
An example of intramolecular Sonogashira-Hagiwara coupling to synthesize an enediyne skeleton. The yield is somewhat low (43% at the highest).
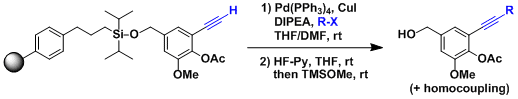
Being reasonably robust and high yielding, the reaction can be used for solid supported substrates. It is also used commonly in combinatorial synthesis and compound library construction.
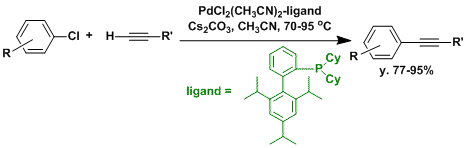
There has been remarkable advancement in palladium catalyzed cross coupling chemistry in recent years. It is now possible to use relatively less reactive aryl bromides and chlorides as substrates using bulky and electron-rich phosphine ligands.[5,6]
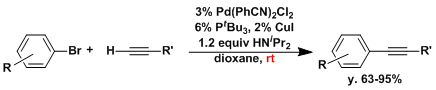
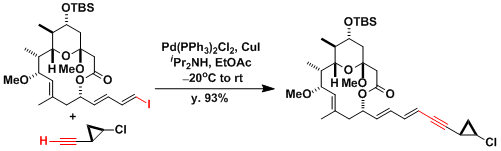
An application to the synthesis of callipeltoside.[7]
-
Experimental Procedure
Sonogashira coupling with pyridine bromide.
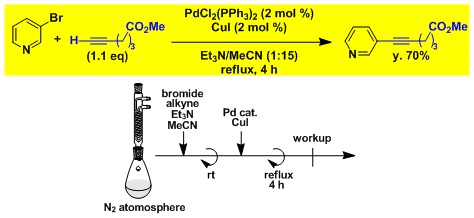
-
Experimental Tips
-
References
[1] Corey, E. J. et al. J. Am.Chem.Soc. 1988, 110, 649. DOI: 10.1021/ja00210a083
[2] Zhang, Q. et al. J. Org. Chem. 2003, 65, 7977. DOI: 10.1021/jo000978e
[3] Dai, W-M.; Wu, A. TetrahedronLett. 2001, 42, 81. DOI:10.1016/S0040-4039(00)01890-6
[4] Liao, Y. et al. Tetrahedron Lett. 2001, 42, 1815. DOI:10.1016/S0040-4039(01)00052-1
[5] Buchwald, S. L. et al. Org. Lett. 2000, 2, 1729. DOI: 10.1021/ol0058947
[6] Buchwald, S. L. et al. Angew. Chem., Int. Ed. 2003, 42, 5993. DOI:10.1002/anie.200353015
[7] Paterson, I.; Davies, R. D. M.; Marquez, R. J. Angew. Chem. Int. Ed. 2001, 40, 603. [abstract]
-
Related Books
[amazonjs asin=”3527305181″ locale=”US” title=”Metal-Catalyzed Cross-Coupling Reactions (2 Volume Set)”]
[amazonjs asin=”3527319379″ locale=”US” title=”Modern Arylation Methods”]
[amazonjs asin=”3540239820″ locale=”US” title=”Palladium in Organic Synthesis (Topics in Organometallic Chemistry)”]
[amazonjs asin=”3540421750″ locale=”US” title=”Cross-Coupling Reactions: A Practical Guide (Topics in Current Chemistry)”]