- Reagent Availability
- Experimental Ease
- Generality
- Criteria #4
- Criteria #5
-
General Characteristics
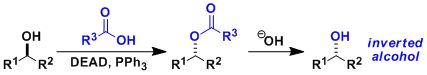
-A secondary alcohol reacts with diethyl azodicarboxylate (DEAD), triphenylphosphine (Ph3P), and benzoic acid to give a corresponding benzoyloxy derivative with the inversion of stereochemistry (SN2 path). The ester product can be hydrolyzed to the corresponding alcohol. Therefore, the Mitsunobu reaction is a useful way to effect stereoinversion of secondary alcohols.
-The reaction works under mild conditions and is used frequently in the synthesis of natural products and other complex compounds.
-A downside is the formation of many byproducts, which sometimes makes TLC monitoring and product purification difficult.
-
General References
・Mitsunobu, O.; Yamada, M.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 1967, 40, 935. doi:10.1246/bcsj.40.935
・Mitsunobu, O.; Yamada, M. Bull. Chem. Soc. Jpn. 1967, 40, 2380. doi:10.1246/bcsj.40.2380
・Review: Mitsunobu, O. Synthesis 1981, 1.
・Review: Hughes, D. L. Org. React. 1992, 42, 335.
・Review: Dandapani, S.; Curran, D. P. Chem. Eur. J. 2004, 10, 3130. DOI: 10.1002/chem.200400363
・Review: Dembinski, R. Eur. J. Org. Chem. 2004, 2763. DOI: 10.1002/ejoc.200400003
・But,T. Y. S.; Toy, P. H. Chem. Asian. J. 2007, 2, 1340. doi:10.1002/asia.200700182
・Swamy, K. C. K.; Kumar, N. N. B.; Balaraman, E.; Kumar, K. V. P. P. Chem. Rev. 2009, 109, 2551.
-
Reaction Mechanism
Since the basicity of the DEAD-Ph3P adduct is low, the nucleophile needs to have a reasonably acidic proton (pKa<13). If one wants simply to have stereoinversion, the use of more acidic p-nitrobenzoic acid instead of benzoic acid is an option as it tends to give higher yield (ref: Chirality 2000, 12, 346).
-
Examples
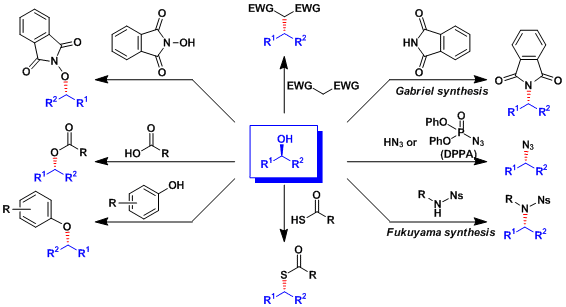
As summarized below, nucleophiles other than carboxylic acid can be used to synthesize a variety of products.
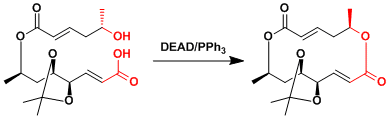
 The Mitsunobu reaction is a valuable tool to synthesize macrolactones.
The Mitsunobu reaction is a valuable tool to synthesize macrolactones.
 Tsunoda reagent: Allows weaker acids (having pKa>13) to react.[1]
Tsunoda reagent: Allows weaker acids (having pKa>13) to react.[1]

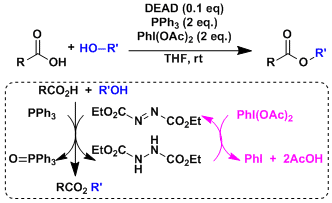
With PhI(OAc)2 as the re-oxidant, DEAD can be used in substoichiometric amount.[2]
IPNBSH reagent developed by Movassaghi is used to deoxygenate alcohols (Movassaghi deoxygenation).
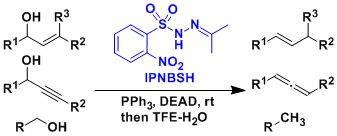
-
Experimental Procedure
Stereoinversion of a congested secondary hydroxyl group.[3]
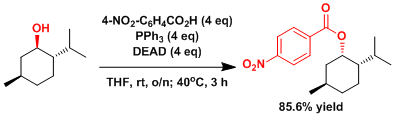
ADD EXPERIMENTAL LATER
-
Experimental Tips
-
References
[1] Tunoda, T. et al. Tetrahedron Lett. 1995, 36, 2529.; ibid, 1996, 37, 2463. DOI: 10.1016/0040-4039(96)00318-8 ; 10.1016/0040-4039(96)00319-X
[2] But, T. Y. S.; Toy, P. H. J. Am. Chem. Soc. 2006, 128, 9636. DOI: 10.1021/ja063141v
[3] Dodge, J. A.; Nissen, J. S.; Presnell, M. Org. Synth. 1996, 73, 110. [PDF]
-
Related Books