Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
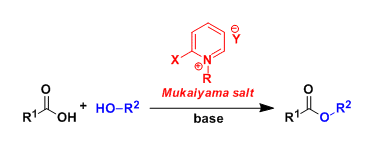
First introduced in 1970’s, 2-halo-N-alkylpyridinium salts are called the Mukaiyama reagent and used for condensation reactions such as esterification and amide bond formation.
-
General References
・Narasaka, K.; Maruyama, K.; Mukaiyama, T. Chem. Lett. 1978, 885. doi:10.1246/cl.1978.885
・Review for macrolactonization: Parenty, A.; Moreau, X.; Campagne, J.-M. Chem. Rev. 2006, 106, 911. DOI: 10.1021/cr0301402
-
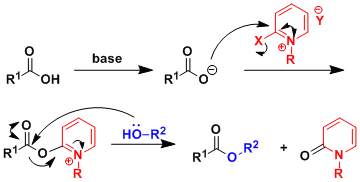
Reaction Mechanism
-
Examples
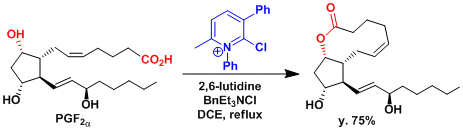
The synthesis of prostaglandin F-lactone.[1]
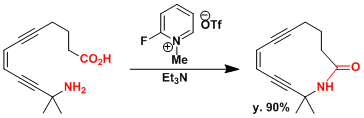
An application to macrolactamization.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Narasaka, K.; Maruyama, K.; Mukaiyama, T. Chem. Lett. 1978, 885. doi:10.1246/cl.1978.885
-
Related Reactions
- Protection of Carboxylic Acid
- Condensation Reagent
- Keck Macrolactonization
- Fischer-Speier Esterification
- Acyl Protective Group
- Yamaguchi Macrolactonizaion
- Mukaiyama Redox Condensation
- Corey-Nicolaou Macrolactonizaion
- Mitsunobu Reaction
-
Related Books
-
External Links