- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
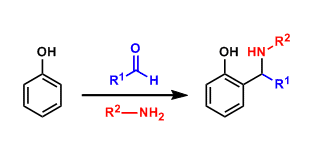
The three component condensation reaction among aldehydes, amines, and phenols is known as the Betti reaction. This reaction is the phenol version of the Mannich reaction.
-
General References
- Betti, M. Gazz. Chim. Ital. 1900, 30 II, 301.
- Betti, M. Gazz. Chim. Ital.1903, 33 II, 2.
- Pirrone, F.Gazz. Chim. Ital.1936, 66, 518.
- Pirrone, F. Gazz. Chim. Ital.1937, 67, 529.
- Phillips, J. P. Chem. Rev.1956, 56, 271. DOI: 10.1021/cr50008a003
- Phillips, J. P.; Barrall, E. M. J. Org. Chem.1956,21, 692. DOI: 10.1021/jo01112a606
-
History
In 1900, the Italian chemist Mario Betti reported the synthesis of a condensation product from the reaction of 2-naphthol with benzaldehyde and an amine. This discovery showed that 2-nathphol was a good nucleophile and the reaction later came to be called the Betti reaction. The products are called the Betti base.
-
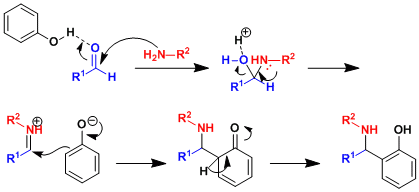
Reaction Mechanism
-
Examples
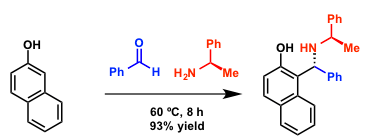
An example in which an optically active amine is used.[1]
-
Experimental Procedure
Synthesis of a Betti base.[1]
A mixture of 2-naphthol (0.72 g, 5.0 mmol), benzaldehyde (0.64 g, 6.00 mmol) and (R)-(+)-1-phenylethylamine (0.64 g, 5.25 mmol) was stirred at 60ºC for 8 h under a nitrogen atmosphere. Following the progress of the reaction by TLC and 1H NMR, it can be seen that the formation of the product occurs in the first two hours, but the inital d.e. (44% at 2h)increases in time (98% at 8 h) with the formation of a solid and crystalline reaction mixture. The reaction mixture was triturated at room temperature with EtOH (5 mL). The white crystals separated were collected and washed with EtOH (3×3 mL). The crystaline white residue, purified by crystallization from EtOAc/hexane, gave the pure product (1.64 g, 4.65 mmol, yield 93%).
-
Experimental Tips
-
References
[1] Palmieri, G. Tetrahedron: Asymmetry 2000, 11, 3361-3373. DOI: 10.1016/S0957-4166(00)00290-1
-
Related Reactions
- Kabachnik-Fields Reaction
- Biginelli Reaction
- Petasis Reaction
- Strecker Amino Acid Synthesis
- Mannich Reaction
-
Related Books
[amazonjs asin=”B008CM2FVA” locale=”US” title=”Enantioselective Organocatalyzed Reactions I: Enantioselective Oxidation, Reduction, Functionalization and Desymmetrization”]
-
External Links