- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
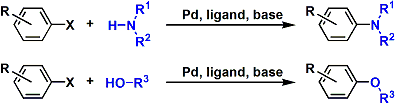
-The palladium-catalyzed cross coupling between aryl halides/triflates and amines/alkoxides is known as the Buchwald-Hartwig reaction. The use of bidentate ligands (e.g. dppf, BINAP) and a strong base is important for facilitating the catalytic cycle.
-This is one of the very few reactions that allow for the direct synthesis of aryl amines and aryl ethers, both of which can not be accessed by simple nucleophilic substitution.
-The demand for multi-functionalized aromatic compounds is high in pharmaceutical, materials, and a number of other fields. The Buchwald-Hartwig coupling is one of the most practical reactions developed in recent decades.
-
General References
・Paul, F.; Patt, J.; Hartwig, J. F. J. Am. Chem. Soc. 1994. 116, 5969. DOI:10.1021/ja00092a058
・Guram, A. S.; Buchwald, S. L. J. Am. Chem. Soc. 1994. 116, 7901. DOI:10.1021/ja00096a059
・Louie, J.; Hartwig, J. F. Tetrahedron Lett. 1995, 36, 3609. doi:10.1016/0040-4039(95)00605-C
<Review>
・Wolfe, J. P.; Wagaw, S.; Marcoux, J. F.; Buchwald, S. L. Acc. Chem. Res. 1998, 31, 805. DOI: 10.1021/ar9600650
・Harwig, J. F. Acc. Chem. Res. 1998, 31, 852.
・Hartwig, J. F. Angew. Chem. Int. Ed. 1998, 37, 2046. [abstract]
・Hartwig, J. F. Pure. Appl. Chem. 1999, 71, 1417. doi:10.1351/pac199971081417
・Prim, D. A.; Campange, J.-M.; Joseph, D.; Andrioletti, B. Tetrahedron 2002, 58, 2041. doi:10.1016/S0040-4020(02)00076-5
・Hartwig, J. F. Synlett 2006, 1283. DOI: 10.1055/s-2006-939728
・Hartwig, J. F. Acc. Chem. Res. 2008, 41, 1534. doi:10.1021/ar800098p
・Surry, D. S.; Buchwald, S. L. Angew. Chem. Int. Ed. 2008, 47, 6338. doi:10.1002/anie.200800497
・Surry, D. S.; Buchwald, S. L. Chem. Sci. 2011, 2, 27. doi:10.1039/c0sc00331j
-
Reaction Mechanism
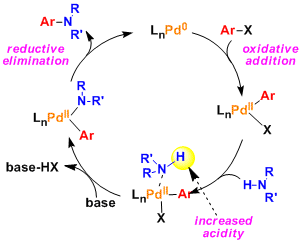
-
Examples
An example of the reaction using relatively unreactive aryl chlorides.[1]
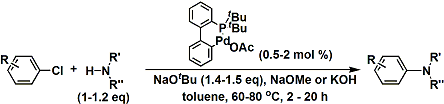
The large scale synthesis of BINAP[2]: Under similar reaction conditions, phosphorus- and sulfur-based groups can be introduced. Strong bases are unnecessary in those cases.
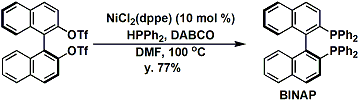
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Zim, D.; Buchwald, S. L. Org. Lett. 2003, 5, 2413. DOI: 10.1021/ol034561h
[2] Org. Synth. 2000, 76, 6.
-
Related Books
[amazonjs asin=”3527305181″ locale=”US” title=”Metal-Catalyzed Cross-Coupling Reactions (2 Volume Set)”]
[amazonjs asin=”3527319379″ locale=”US” title=”Modern Arylation Methods”]
[amazonjs asin=”0470850329″ locale=”US” title=”Palladium Reagents and Catalysts: New Perspectives for the 21st Century”]
[amazonjs asin=”3540239820″ locale=”US” title=”Palladium in Organic Synthesis (Topics in Organometallic Chemistry)”]
[amazonjs asin=”3540421750″ locale=”US” title=”Cross-Coupling Reactions: A Practical Guide (Topics in Current Chemistry)”]

Pingback: Arylation at Lysine Residues | Chem-Station Int. Ed.