- Generality
- Reagent Availability
- Experimental User Friendliness
- Scope of Reported Examples
- Criteria #5
-
General Characteristics
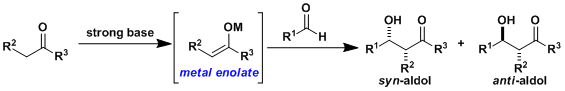
-In cross aldol reactions, the donor carbonyl compound is deprotonated completely by a strong base like LDA to preform its metal enolate, which prevents self-condensation upon reacting with the acceptor to generate the cross aldol product.
-Depending on its geometry, the enolate reacts with the acceptor aldehyde or ketone stereospecifically to give either syn or anti product.
-Various metals can be used via transmetallation including Li, Na, Mg, Zn, B, Al, and Ti, among which silyl and tin enolates can be isolated and purified. In particular, the aldol addition using silyl enolates is known as the Mukaiyama aldol reaction.
-There are many total syntheses in which cross aldol reactions are used to couple large molecular fragments. Application examples and other detailed information are available in many journals and reviews.
-
General References
・Heathcock, C. H. Comprehensive Organic Synthesis 1991, 2, 133.
・Kim, B. M. et al. Comprehensive Organic Synthesis 1991, 2, 239.
・Paterson, I. Comprehensive Organic Synthesis 1991, 2, 301.
・Mahrwald, R. ed. Modern Aldol Reactions Wiley-VCH, 2004
・Review: Palomo, C.; Oiarbide, M.; Garcia, J. M. Chem. Eur. J. 2002, 8, 36. [abstract]
・Review: Palomo, C.; Oiarbide, M.; Garcia, J. M. Chem. Soc. Rev. 2004, 33, 65. DOI: 10.1039/b202901d
・Review: Schetter, B.; Mahrwald, R. Angew. Chem. Int. Ed. 2006, 45, 7506. doi: 10.1002/anie.200602780
-
Reaction Mechanism
The Zimmerman-Traxler six-membered transition state model (J. Am. Chem. Soc. 1957, 79, 1920.) is generally used to explain the stereochemistry. The substituent of the aldehyde is considered stable pointing equatorially, therefore the stereochemical outcome is determined by the geometry of the enolate: Z-enolates give syn-aldols and E-enolates give anti-aldols.
In general, metals capable of forming a strong M-O bond (hard and chelating metals) form a “tight” transitions state and tend to make the reaction more stereoselective.
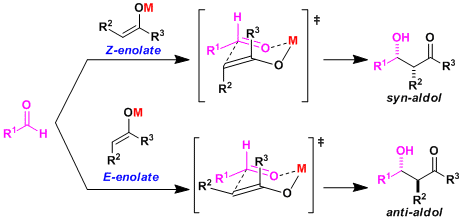
The addition of strongly coordinating solvents such as HMPA increases the polarity of the metal enolate and the reactivity. But since it disrupts the formation of six-membered transition state, the stereoselectivity will be influenced by other factors.
-
Examples
In the enolate formation of unsymmetrical ketones, the regioselectivity can often be controlled by selecting appropriate conditions (between kinetically and thermodynamically controlled conditions).
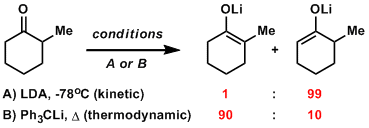
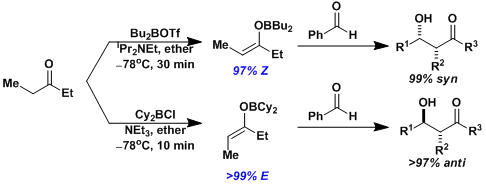
With the short B-O bond length, boron enolates form “tight” six-membered transition states and tend to give higher stereoselectivity than lithium enolates.
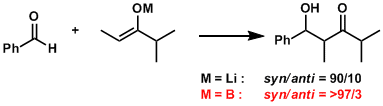
Boron enolates can be prepared in either E or Z form depending on the type of reagent.[1]
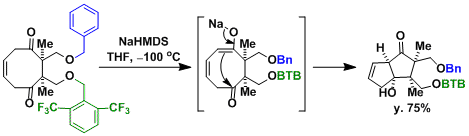
Synthesis of merrilactone A[2]: Desymmetrization by intramolecular aldol reaction.
When one of the carbonyl reactants is incapable of forming an enolate (e.g. HCHO, ArCHO, Ar2O), crossed aldol reactions work easily (the Claisen-Schmidt reaction).[3]
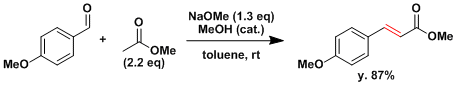
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Brown, H. C.; Dhar, R. K.; Bakshi, R. K.; Pandiarajan, P. K.; Singaram, B. J. Am. Chem. Soc. 1989, 111, 3441. DOI: 10.1021/ja00191a058
[2] (a) Inoue, M.; Sato, T.; Hirama, M. Angew. Chem. Int. Ed. 2006, 45, 4843. doi:10.1002/anie.200601358 (b) Inoue, M.; Lee, N.; Kasuya, S.; Sato, T.; Hirama, M.; Moriyama, M.; Fukuyama, Y. J. Org. Chem. 2007, 72, 3065. DOI: 10.1021/jo0700474
[3] (a) Schmidt, J. G. Ber. 1880, 13, 2342. (b) Claisen, L. Ber. 1890, 23, 976.
-
Related Books
[amazonjs asin=”3527307141″ locale=”US” title=”Modern Aldol Reactions (2 Volume Set)”]
[amazonjs asin=”3527298711″ locale=”US” title=”Modern Carbonyl Chemistry”]
[amazonjs asin=”0198559577″ locale=”US” title=”Stereoselectivity in Organic Synthesis (Oxford Chemistry Primers, 63)”]