For Spotlight Research, we had the pleasure of interviewing Prof. Ryota Kabe from Center for Organic Photonics and Electronics Research (OPERA), Kyushu University. OPERA is a leading research institute working at the frontiers of organic semiconductors.
Although much progress have been made in the field of organic semiconductors, the development of organic luminescence devices has remained as a challenge. The reason is that, for practical applications, these devices must produce persistent emission, which requires the stabilization of the excited electronic state to attain longer luminescence lifetime. This time, we introduce an exciting work that utilizes a very simple system, but has the potential to revolutionize organic electronic materials. The work was published in Nature, and the original paper can be found from the following link.
“Organic long persistent luminescence”
Ryota Kabe & Chihaya Adachi
Nature, 2017, 550, 384−387. DOI: 10.1038/nature24010
You are wondering why this work is selected?
Because the title is unusually short. The author (spectol21) is attracted to papers with short titles as they prove to us how simple the inspiration behind novel discoveries can be. I feel my eyes are refreshed at the sight of the title.
The lab’s principal investigator Prof. Chihaya Adachi has commented on Prof. Kabe and his work,
Before Ryota Kabe became deeply interested in organic photo-electronics, he was studying coordination chemistry. Back then, he has accumulated broad knowledge and research experience of many disciplines ranging from device processing to optoelectronic property analysis. He is indeed a young and spirited researcher ready to explore the interplay of chemistry and physics. The research on organic long persistent luminescence (OLPL) materials has evolved during the years. The first generation as studied by Dr. Shuzo Hirata (currently assistant professor at Tokyo Institute of Technology) utilizes a solid matrix to reduce thermal quenching. Now, we see the second generation made possible by Kabe. The working principle of this new OLPL material is charge separation. I think its creation is largely indebted to the dynamic atmosphere at OPERA full of diverse researchers and Kabe’s passion towards LPL materials. In the future, I expect that more young researchers will join OLPL research, pushing the frontiers of this exciting field.
We had the pleasure of interviewing Prof. Kabe and let’s see what messages he has for us!
Q1. What is your research about? Can you briefly walk us through your work?
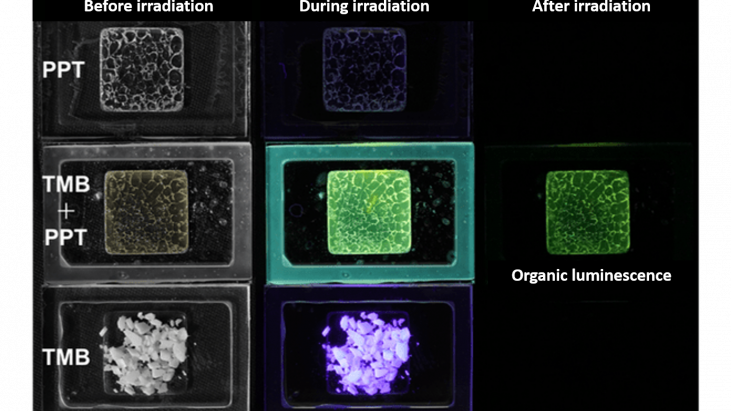
In one sentence, we made a completely organic luminescence material that can emit light for as long as 1 hour at room temperature, utilizing a long-lived charge separated state between organic molecules.
Luminescent materials can store light energy and gradually release it over a long period of time. Exploiting their ability to emit light in the absence of electrical source, luminescent materials are commonly used as glow-in-the-dark paint for watches and emergency signs. Up to now, all commercialized long persistent luminescent (LPL) materials are based on inorganic compounds which usually contain rare elements. The inorganic compound is produced by heating to over 1000 ºC and since the product is insoluble, grinding and dispersing become necessary.
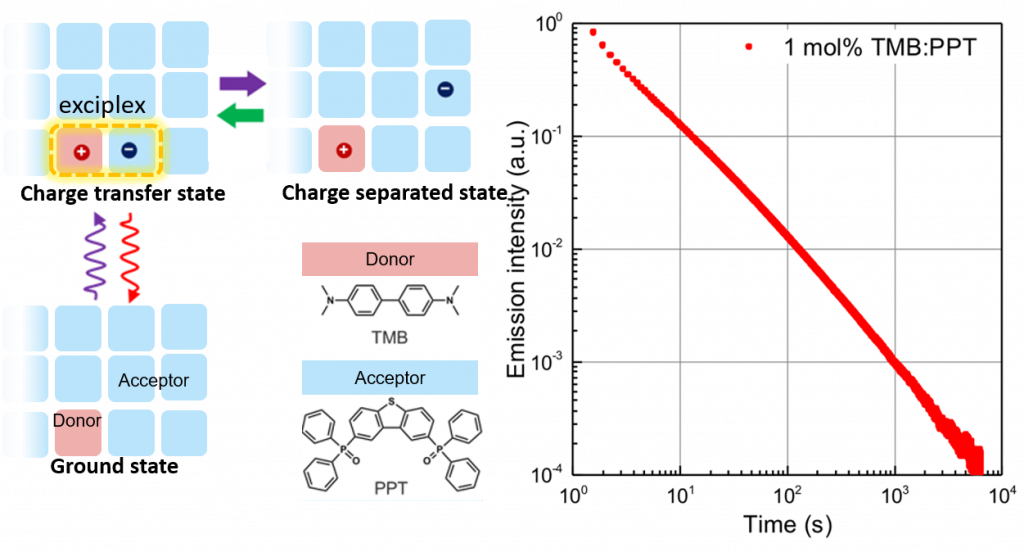
By contrast, our organic LPL (OLPL) system is simply the blend of two organic compounds, so it is soluble and easy to process. The organic system we use contains 1% of electron-donating molecule (donor) TMP and 99% of electron-accepting molecule (acceptor) PPT. When the material is illuminated by light, the electron in the donor is first promoted to the excited state and then it transfers to the acceptor. Receiving this extra electron, the acceptor forms an radical anion and the electron is able to hop between acceptors, moving away from the donor to yield a charge-separated state. The mechanism of light absorption and charge separation at the donor-acceptor interface is the same as the working principle of organic photovoltaics (OPVs).
However, there are also several differences between OPVs and OLPLs. Aside from the fact that OLPLs have no electrode to conduct the electrons, a more fundamental difference is that the radical anion in OLPL is highly stabilized owing to the acceptor structure. Therefore, the charge-separated state has time to diffuse in the system. Eventually, it comes back to the donor-acceptor interface and the two charged molecules recombine to form an excited complex (exciplex). The exciplex emits at longer wave lengths compared to excited donor or acceptor molecules. The mechanism of such light emission upon charge recombination is similar to that of organic LEDs. In fact, our group has also reported on the formation of excited triplet state via charge recombination and we have shown that light exciplex emission is attributed to thermally assisted delayed fluorescence (TADF) [1].
As explained above, the light energy is first converted and stored in excited charge-separated states which eventually recombines and generates ecicomplex emission. Through this process, long persistent luminescence is achieved. Here, charge recombination is the rate-determining step, that’s why the LPL intensity does not show a single exponential decay but is instead inversely proportional of time, as indicated by the slope of the log-log plot. Although the phenomena of luminescence are more comprehensible, the highlight of this research is the realization of a room-temperature stable charge-separated state from a simple organic system.
Q2. What is creative about this research? How did you realize your idea?
Although we came up with the idea of prolonging light emission by storing the energy in the charge-separated state about 3 years ago when we just started the ERATO project, we didn’t know how to realize it.
What we knew at the time was the the last step must be radiative emission from the excited states, so we started with thinking of ways to prevent non-radiative deactivation. Many researchers have worked on this topic and for example one idea is to use a polymer material called poly(methyl methacrylate) and in recent years using steroid derivatives is also reported. In particular, Dr. Hirata has contributed to the detailed elucidation on the unique characters of such materials [2].
However, the existing materials that suppresses non-radiative deactivation are all electrical insulators, and therefore they cannot be used in organic semiconductors, including OLEDs and OPVs where a triplet exciton is formed. So we began with considering if this mechanism can be extended to organic semiconductors. Then our Ph.D. student Naoto Noutsuka pointed out that if we can cut the heat transfer pathway in the host matrix, we may be able to suppress non-radiative deactivation [3].
We figured if we employ charge separation and subsequent recombination, we may achieve long luminescence lifetime impossible for direct excited-stated emission. So we started to put together molecules that have a charge-separated state and built many complicated systems from multiple molecules. However, none of them worked. Our inspiration came while discussing with Prof. Katsumi Tokumaru, and reading the pioneering works on two-photon ionization by Prof. Masahide Yamamoto and Prof. Hideo Ohkita (Kyoto Univerisity) [4]. With some “serendipity”, we finally got the idea and succeeded with the extremely simple system containing only 2 molecules. (I can’t talk about the “serendipity” part because it’s related to an unpublished work.)
Q3. What do you think is the major challenge of this research? How did you overcome it?
In most cases, emission intensity decreases exponentially with time and the luminescence lifetime is defined based on this model. However, the rate-determining step of our organic luminescent system is recombination of the charge-separated state, so the kinetics does not follow an exponential decay. For this reason, the common standards are not compatible with our system, and we are still searching for appropriate standards to evaluate organic luminescence behavior.
In addition, although there are many instruments for spectroscopic studies and characterization of transient emission behaviors, they are not designed for persistent luminescence. (In our system, the emission profile depends not only on the excitation intensity, but also the irradiation time.) We could not use available instruments for OLPL materials and had to start from building our own evaluation system.
Q4. What kind of chemistry do you want to engage in?
I want to engage myself with building new ideas from the ground up, like going from 0 to 1.
Q5. As a final remark, can you give the readers some advice or message?
In this research, we have proposed a new mechanism for organic luminescence. What we actually did is as simple as blending up two existing compounds and measure the emission. Whoever understands the mechanism can imagine it happen.
Initially, we exhausted ourselves thinking about complicated systems, yet what turns out to work the best was the simplest classical system of electron donor and acceptor. There are many other examples where unique functions come from extremely simple structures, for instance organic ferroelectricity (chronic acid) [5] and organosuperelasticity (terephthalamide) [6]. They are so simple that we have overlooked them. If we can think out of the box a bit, we may make new discoveries from common objects around us.
As a final remark, I express sincere gratitude for the fantastic research environment at OPERA, the instructions from Prof. Adachi and numerous advices from my ERATO advisors Prof. Katsumi Tokumaru, Prof. Masahiro Kotani and Prof. Hiroyuki Sasabe.
References
- Goushi, K. Yoshida, K. Sato, C. Adachi, Nat. Photonics 2012, 6, 253. DOI: 10.1038/nphoton.2012.31
- S. Hirata, Adv. Opt. Mater. 2017, 5, 1700116. DOI: 10.1002/adom.201700116
- N. Notsuka, R. Kabe, K. Goushi, C. Adachi, Adv. Funct. Mater. 2017, 27, 1703902. DOI: 10.1002/adfm.201703902
- H. Ohkita, W. Sakai, A. Tsuchida, M. Yamamoto, Macromolecules 1997, 30, 5376. DOI: 10.1021/ma970585q
- S. Horiuchi, Y. Tokunaga, G. Giovannetti, S. Picozzi, H. Itoh, R. Shimano, R. Kumai, Y. Tokura, Nature 2010, 463, 789. DOI: 10.1038/nature08731
- S. Takamizawa, Y. Miyamoto, Angew. Chem., Int. Ed. 2014, 53, 6970. DOI: 10.1002/ange.201311014
Biography of Researcher
Ryouta Kabe
Institute: Center for Organic Photonics and Electronics Research (OPERA), Kyushu University
Biography:
2005/03 B.Sc., Kansai University (Prof. Yamauchi)
2007/03 Ph.D., Osaka University(Prof. Fukuzumi, Prof. Ogo )
2008/04 – 2010/03 Doctor Course Student Fellowship, Japan Society for the Promotion of Science
2009/08 – 2009/11 Visiting researcher, University of Southern California(Prof. M. E. Thompson)
2010/03 Post doc. Kyushu University(Prof. Ogo, Prof. Adachi)
2010/04 – 2011/03 Visiting researcher, Bowling Green State University(Prof. P. Anzenbacher Jr.)
2011/04 – 2014/01 Post Doctor Fellowship, Japan Society for the Promotion of Science
2011/04 – 2012/08 Visiting researcher, Max Planck Institute for Polymer Research(Prof. K. Müllen)
2014/02 –now Assistant professor, Center for Organic Photonics and Electronics Research (OPERA), Kyushu University
Project leader, JST ERATO ADACHI Molecular Exciton Engineering Project
This article is originally written in Japanese by spectol21 (Japanese version) and translated by Elise Chen.