Spotlight Research is delivered by Dr. Kousuke Kuroda, assistant professor from the Laboratory for Bioactive Substances Engineering, Kanezawa University.
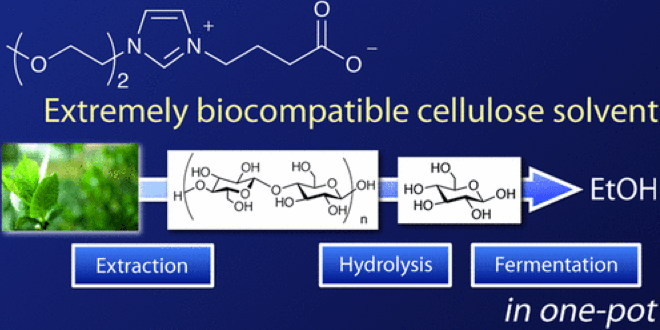
Waste biomass is a prospective energy source of the future. Here at Kanezawa University, the Chemical Reaction Engineering Laboratory (Kenji Takahashi group) studies how to employ ionic liquid, supercritical fluid, plasma and microwave for harnessing biomass energy. As a member of the group, Dr. Kuroda recently developed a new ionic liquid and succeeded with dissolving biomass without destroying the cell membranes (The top image is from the original paper shown below).
Kosuke Kuroda, Heri Satria, Kyohei Miyamura, Yota Tsuge, Kazuaki Ninomiya, Kenji Takahashi,
”Design of Wall-Destructive but Membrane-Compatible Solvents” J. Am. Chem. Soc.2017, 139, 16052-16055 DOI:10.1021/jacs.7b08914
Q1. What is your research about? Can you briefly walk us through your work?
Although bio-ethanol is an alternative fuel for gasoline, currently it is produced from corn and other foods, and some worry that the production of bio-ethanol may cause food shortage. Hence, it is more desirable to make ethanol from waste biomass, predominantly cellulose-based matters such as grass, wood, paper cup and disposable chopsticks. However, as the available solvents for cellulose are highly toxic for microorganisms, they must be removed before fermentation. Because the process of solvent removal costs even more energy than that stored in biofuel, the net energy production becomes negative, which is a serious obstacle for utilizing these resources.
Our group developed a novel biomass solvent composed of carboxylate-type zwitterionic liquid which shows both high solvation ability and low toxicity towards microorganisms. With this new solvent, microorganisms can survive and produce ethanol in concentrated solvent environment, lowering the energy cost and making it feasible to produce biofuel from waste biomass.
Q2. What is creative about this research? How did you realize your idea?
We had long known that the existing biomass solvents were highly toxic for microorganisms and everyone thought it impossible to dissolve cellulose without damaging the fragile cell membrane. In spite of this, we kept on studying the interaction between ionic liquid and the cell membrane and sought for the optimal structure of ionic liquid. Eventually, we found that carboxylate-type ionic solvent as shown above can be used for the purpose.
I received my Ph.D. degree at Tokyo University of Agriculture and Technology (Ohno Nakamura group) where I learned to synthesize ionic liquid and analyze its interaction. After that I joined Takahashi and Nimiya group to study microbial fermentation. This research integrates my two research experiences and I regard it as the compilation of my twenties.
Q3. What do you think is the major challenge of this research? How did you overcome it?
The research itself progressed very smoothly, yet I had a hard time to decide on the title of my paper. Another challenge was the sheer amount of experiments we did. For the title, I debated between focusing on “successful ethanol production” or “novel ionic liquid”. However, Prof. Kiyohiko Igarashi (University of Tokyo) gave us his timely advice “Don’t make it narrow, go broad!” In the end, we decided on the title “Design of Wall-Destructive but Membrane-Compatible Solvents”.
For the experimental part, we really did a lot of experiments. The data we published is not even one tenth of what we actually obtained. I’m really glad that our Ph.D. students Dr. Kyouhei Miyamura and Dr. Heri Satria’s effort brought the project to fruition.
Q4. What kind of chemistry do you want to engage in?
I want to find out more interesting aspects of ionic liquid. Many people have heard of ionic liquid and their general opinion is “Anyway it must be expensive.” It’s true. Although the price has dropped recently, ionic liquid is still expensive compared to usual solvents. However, ionic liquid has its unique charm to compensate for the price: the diversity of structures that can be designed. In this research, we simply joined the cation and the anion with a covalent bond and the ionic liquid we obtained showed a completely different “face”. Likewise, by tuning their structure, I believe we can find out many exclusive applications of ionic liquid. As there are infinite possible structures, there must be many undiscovered “faces” of ionic liquid. I will be happy to find out more of these faces.
Q5. As a final remark, can you give the readers some advice or message?
Ionic liquid is not just for dissolving cellulose. It has many applications in various fields and may be used as the electrolyte in battery, the solvent media of enzymes and the solvent for liquid-liquid extraction. Ionic liquid may contribute to the development of many sciences. If you are interested, welcome to join our research. Let’s share the fun together!
Biography of Researcher
 Kousuke Kuroda
Kousuke Kuroda
Institution: Laboratory for Bioactive Substances Engineering, Kanezawa University.
Current research: Development of new ionic liquid and biomass utilization
Biography:
2012/4-2014/9 Doctor Course Student Fellowship, Japan Society for the Promotion of Science
2014/9 Ph.D., Biotechnology and Life Science, Tokyo University of Agriculture and Technology (Ohno and Nakamura group)
2014/10-2017/9 Assistant professor, Chemical Reaction Engineering Laboratory, Kanezawa University, (Kenji Takahashi group)
2015/10-now Assistant Professor, Laboratory for Bioactive Substances Engineering, Kanezawa University
Group website: http://ionicliquid.w3.kanazawa-u.ac.jp
The article is originally written by Megu (Japanese version), and translated by EliseChen.