This Spotlight Research is presented by Dr. Satoshi Okada from Nakamura group at the Department of Chemistry, University of Tokyo. Currently, Dr. Satoshi Okada is a researcher at the R&D Center for Marine Biosciences, Japan Agency for Marine-Earth Science and Technology.
The Nakamura group leads research on electron microscopic imaging of single molecules. The group also studies the catalytic functions of ubiquitous metals and the fabrication of organic photovotaics.
Recently, the group led by Prof. Eiichi Nakamura, Prof. Koji Harano and the group led by Prof. Kaoru Yamanouchi are studying the stochastic chemical kinetics by directly observing molecules under microscopes, and they have found their observations to correlate well with quantum mechanical predictions. The research is reported in J. Am Chem. Soc.
Direct Microscopic Analysis of Individual C60 Dimerization Events: Kinetics and Mechanisms
Okada, S.; Kowashi, S.; Schweighauser, L.; Yamanouchi, K.; Harano, K.; Nakamura, E. J. Am. Chem. Soc. 2017, 139, 18281–18287.
DOI: 10.1021/jacs.7b09776
We have interviewed Dr. Okada and the principal investigator Prof. Nakamura also gave us some remarks.
Dr. Okada joint our lab as an undergraduate student and we worked together for six years. He started this project in his third year of doctorate.
We had the data of dimerization of fullerenes from 2010, but one day as I rechecked the data, it happened to me that although the reaction seems random, universally it may be governed by the first order kinetics. I mentioned the idea to Okada and thanks to his attentiveness, we saw some progress in two weeks.
However, it became harder after that. At first, we knew nothing more than the Eyring equation, thankfully my colleague Prof. Yamanouchi has taught us about the RRKM theory and Dr. Kowashi helped to achieve variable-temperature conditions. We had collaborated with Prof. Harano for two years and finally got to where we are. I believe this experience will help Okada tremendously as he embarks on the research of marine chemistry afterwards.
Please enjoy the following interview and take a look at the paper if you like.
Q1. What is your research about? Can you briefly walk us through your work?
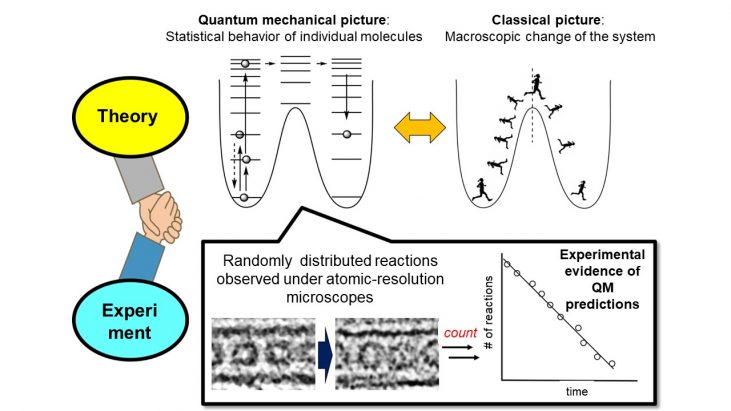
In chemistry, we study molecules by measuring the averaged properties of a bulk sample using experimental techniques such as spectroscopy. The Nobel prize in 2017 is no exception: many frozen biomolecules are observed by cryo-electron microscopy and an averaged image is taken to obtain high-resolution data. On the other hand, the viewpoints of theoretical and computational chemistry are built upon individual molecules.
So can we gain insights of the bulk properties from direct observation of single molecules? For example, can we know the rate law from directly observing the reaction of individual molecules? To answer this question, it is necessary to take a number of videos of chemical reactions at atomic resolution.
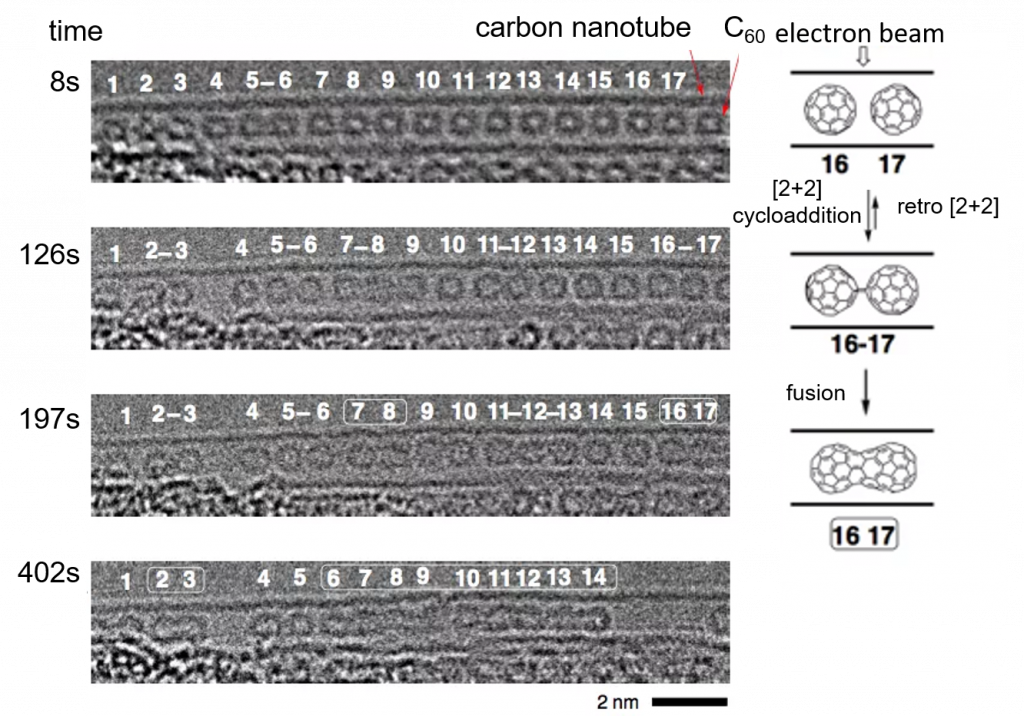
The Nakamura group has developed the technique of taking such atomic-resolution videos of molecules by confining them inside carbon nanotubes (CNTs). However, in order to “see” reactions, we also need to figure out the molecules to observe and the mechanism of the reaction.
Picture adopted from the original paper
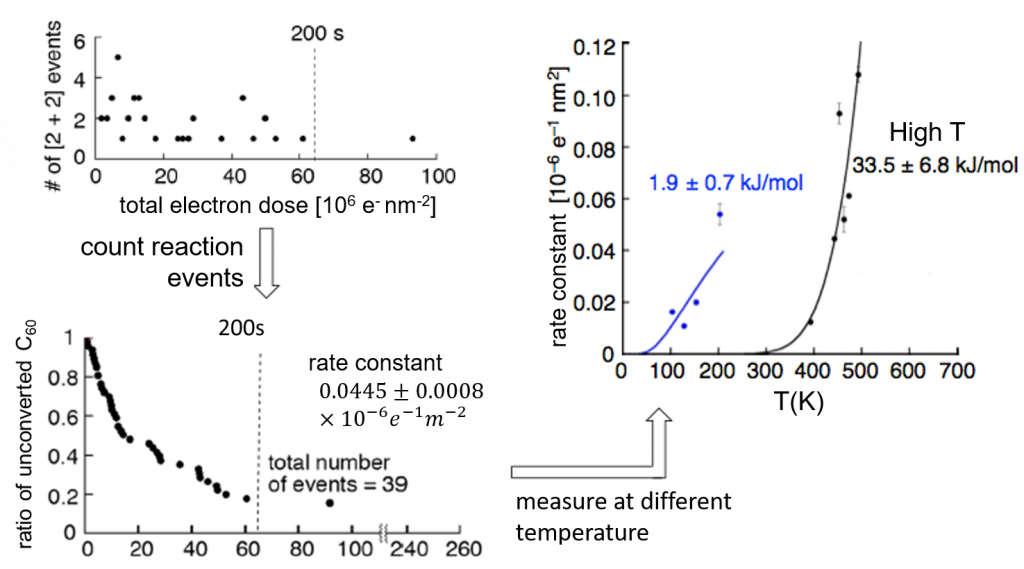
In this study, we chose the [2+2] cycloaddition of fullerenes inside CNTs as the model reaction and observed it under TEM. Through the application of the RRKM theory, which is based on the transition state theory of single molecules, we proved that it is possible to calculate the activation energy by looking at as few as dozens of molecules. Moreover, we found that the activation energy at above 400K and below 200K are different for about 20 times, implying different reaction pathways. If you want to know the details, please check out our paper.
Picture adopted from the original paper
Q2. What is creative about this research? How did you realize your ideas?
The structure of fullerene dimer was reported in 2010. Since then, we know such phenomena can be observed under variable-temperature TEM. I was discussing with Prof. Nakamura, and we realized that if we know the temperature and the amount of electron beam needed to trigger the reaction, we can calculate the activation barrier. Just to verify the idea, I took some data points from the graph on an old paper and tried to make a new plot.
We immediately saw a curve representative of first order reaction from the plot. I learned it the hard way the importance of re-examining existing data. In the meantime, I thought it very interesting and decided to concentrate on this research.
Q3. What do you think is the major challenge of this research? How did you overcome it?
At first when we began this project, I was a layman at reaction mechanisms and could only rely on textbooks to gain understandings. I was very unsure about whether my ideas on are the right track.
I then consulted Prof. Kaoru Yamanouchi, the expert in this field, and finally I understood the RRKM theory. I also realized that some of my initial thoughts were wrong. It hit me that rather than using crammed knowledge, it is better to ask the experts and learn from them.
Q4. What kind of chemistry do you want to engage in?
I want to broadcast the joy of visualizing things and reveal the importance of interpreting the visible phenomena.
I’m currently studying deep-sea organisms and soft materials at the Research and Development Center for Marine Biosciences. Here, we seek to understand the function of marine organisms from the point of nanostructure instead of genetics. I think high-resolution microscopy is a vital tool for us to study those soft material and understand their nanostructures.
Q5. As a final remark, can you give the readers some advice or message?
As many researchers have said in Spotlight Research, it is important to talk to people in different fields, even though it is harder to communicate ideas across disciplines. So I believe it’s important to have a broader knowledge, not necessarily deep in every field, but we need to know the basics to communicate with other scientists.
It is really satisfactory to take beautiful pictures with TEM and other microscopic techniques. As the old saying goes, seeing is believing, the impact of one microscopic image is usually larger than one spectrum.
However, although it is important to sharpen one’s skills to take beautiful photos, it is no more than craftsmanship. As scientists, we need to understand those images and ask ourselves why we could take such photos and why the structures appear as this or that. It’s important to make hypotheses and test them by experiments.
Last but not least, I express sincere gratitude for the fantastic research environment, the instructions from Prof. Eiichi Nakamura and numerous advices from my Prof. Kaoru Yamanouchi, Prof. Koji Harano. Also special thanks to Ms. Satori Kowashi who did many supplementary experiments with great patience.
Biography of Researcher

Satoshi Okada
Frontier Research Group, R&D Center for Marine Biosciences, Japan Agency for Marine-Earth Science and Technology
Research interest: Nanochemistry of deep-sea environment, Biomineralization
Biography:
2011/03 B.Sc., Department of Chemistry, University of Tokyo (Prof. Eiichi Nakamura)
2012/02-2016/03 Advanced Leading Graduate Course for Photon Science (ALPS), University of Tokyo
2013/10–2014/01 Visiting student, École Supérieure de Physique et de Chimie Industrielles de la Ville de Paris, France (Prof. Ludwik Leibler)
2015/03 Visiting student, Saint Petersburg State University, Russia (Prof. Igor V. Murin)
2016/04-now Frontier Research Group, R&D Center for Marine Biosciences, Japan Agency for Marine-Earth Science and Technology
The article is originally written by webmaster (Japanese version), and translated by EliseChen.