- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
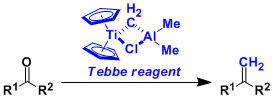
The titanium-based Tebbe reagent is a valuable tool when converting carbonyl groups into exo-olefins.
The reagent can be used for the methylenation of not only aldehydes and ketones, but also esters, lactones, amides, and thioesters, which cannot be achieved by the Wittig reagent.
The high Lewis acidity of the reagent allows it to react with sterically hindered substrates. It is also only weakly basic, thus can react with enolizable substrates with a minimal risk of racemization.
The Tebbe methylenation can be followed by ring closing olefin metathesis when the substrate (the product) contains another olefin in an appropriate position.
-
General References
・ Tebbe, F. N.; Parshall, G. W.; Reddy, G. S. J. Am. Chem. Soc. 1978, 100, 3611. doi:10.1021/ja00479a061
・ Pine, S. H.; Zahler, R.; Evans, D. A.; Grubbs, R. H. J. Am. Chem. Soc. 1980, 102, 3270. DOI: 10.1021/ja00529a076
・ Brown-Wensley, K. A.; Buchwald, S. L.; Cannizzo, L.; Clawson, L.; Ho, S.; Meinhardt, D.; Stille, J. R.; Straus, D.; Grubbs, R. H. Pure Appl. Chem. 1983, 55, 1733. doi:10.1351/pac198355111733
・ Pine, S. H. et al. J. Org. Chem. 1985, 50, 1212. DOI: 10.1021/jo00208a013
・ Pine, S. H. et al. Org. Synth. 1990, 65, 72.
・ Kelly, S. E. Comp. Org. Syn. 1991, 1, 743.
・ Pine, S. H.; Shen, G. S.; Hoang, H. Synthesis 1991, 165. DOI: 10.1055/s-1991-26406
・ Pine, S. H. Org. React. 1993, 43, 1.
・ Beadham, I.; Micklefield, J. Curr. Org. Syn. 2005, 2, 231.
-
Reaction Mechanism
The active species is believed to be the titanocene Schrock carbene (CP2Ti=CH2), which undergoes metathesis with carbonyls to form carbon-carbon double bonds. The formation of particularly strong titanium-oxygen bond is the driving force of the reaction.
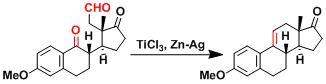
-
Examples
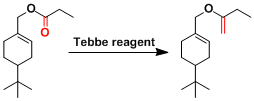
-
Experimental Procedure
The Tebbe reagent can be prepared by mixing titanocene dichloride (Cp2TiCl2) and trimethylaluminum in toluene.
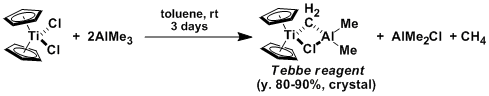
The experiment has to be conducted under inert gas atmosphere as both trimethylaluminum and the Tebbe reagent are pyrophoric.
-
Experimental Tips
-
References
-
Related Books
[amazonjs asin=”352730634X” locale=”US” title=”Modern Carbonyl Olefination: Methods and Applications”]