- Generality
- Reagent Availability
- Experimental User Friendliness
- Historical Significance
- Criteria #5
-
General Characteristics
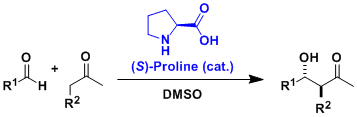
The asymmetric aldol reactions can be catalyzed by simple amino acids such as proline.
The proline-catalyzed intramolecular asymmetric aldol reactions of limited substrates had been known by 1970s. In 2000, List, Barbas, and Lerner reported intermolecular and more general applications. Their work pioneered the development of organocatalysis, now a major subfield of organic chemistry.
-
General References
・ List, B.; Lerner, R. A.; Barbas, C. F., III J. Am. Chem. Soc. 2000, 122, 2395. DOI: 10.1021/ja994280y
・ Notz, W.; List, B. J. Am. Chem. Soc. 2000, 122, 7386. DOI: 10.1021/ja001460v
・ List, B.; Pojarliev, P.; Castello, C. Org. Lett. 2001, 3, 573. DOI: 10.1021/ol006976y
・ Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C. F., III J. Am. Chem. Soc. 2001, 123, 5260. DOI: 10.1021/ja010037z
-
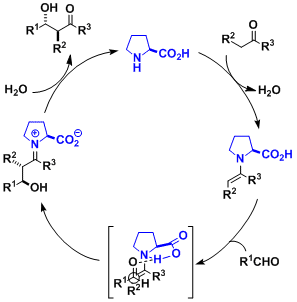
Reaction Mechanism
The catalytic cycle shown below is generally considered operative in most cases. The ketone (the donor) and proline condense to form the nucleophilic enamine intermediate first. The proton of proline’s carboxylic acid is believed to interact with and activate the aldehyde (the accepter), contributing in increasing both selectivity and reactivity.
-
Examples
Organocatalytic crossed-aldol reaction between two different aldehydes has been reported. This has not been achieved by organometallic catalysis (as of April 2011).[1]

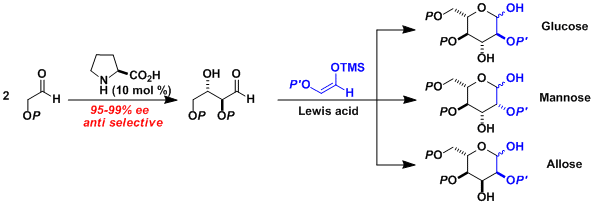
The synthesis of protected hexose derivatives based on stereoselective aldol reaction was reported by MacMillan.[2] This procedure provides the shortest route to these monosaccharides among all other reported stereoselective methods. The partially protected products are well suited for further derivatization and the procedure is compatible with various applications such as the introduction of nitrogen and sulfur atoms as well as 13C labelling.
-
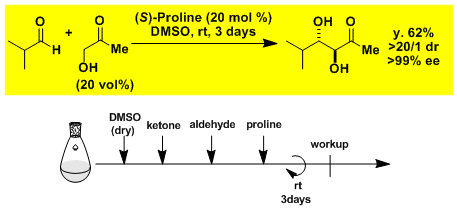
Experimental Procedure
Enantio- and diastereoselective aldol reaction with hydroxyketone as a donor.[3]
-
Experimental Tips
The ketone is usually used in excess in order to shift the equilibrium to the product side.
-
References
[1] Northrup, A. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 6798. DOI: 10.1021/ja0262378
[2] Northrup, A. B.; MacMillan, D. W. C. Science 2004, 305, 1752. DOI: 10.1126/science.1101710
[3] Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C. F., III J. Am. Chem. Soc. 2001, 123, 5260. DOI: 10.1021/ja010037z
-
Related Books
[amazonjs asin=”3527305173″ locale=”US” title=”Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis”]