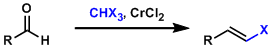- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-The synthesis of alkenyl halides from aldehydes using the gem-dichromium reagent prepared from haloform (CHX3) and CrCl2 is known as the Takai-Uchimoto reaction. Mild reaction conditions thanks to high functional group tolerance of the dichromium reagent are the most beneficial aspect of this reaction. The main products are generally E-alkenes.
-1,1-Dihalides (CR2X2) can be used in place of CHX3.
-
General References
・Takai, K.; Nitta, K.; Uchimoto, K. J. Am. Chem. Soc. 1986, 108, 7408. doi:10.1021/ja00283a046
・Okazoe, T.; Takai, K.; Uchimoto, K. J. Am. Chem. Soc. 1987, 109, 951. doi:10.1021/ja00237a081
・Fürstner, A. Chem. Rev. 1999, 99, 991. doi: 10.1021/cr9703360
・Wessjohann, L. A.; Scheid, G. Synthesis 1999, 1. doi:10.1055/s-1999-3672
-
Reaction Mechanism
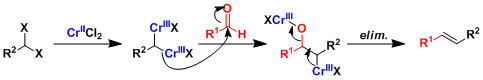
The gem-dichromium species is considered to be the active nucleophile.
-
Examples
The major products are E-alkenes but the selectivity depends on the system.[1]
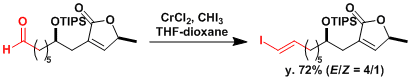
An application to the synthesis of phorboxazole A.[2]
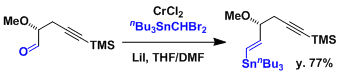
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Hoye, T. R.; Tan, L. Tetrahedron Lett. 1995, 36, 1981. doi:10.1016/0040-4039(95)00207-S
[2] Smith, A. B., III et al. J. Am. Chem. Soc. 2001, 123, 10942. DOI: 10.1021/ja011604l
-
Related Books
[amazonjs asin=”352730634X” locale=”US” title=”Modern Carbonyl Olefination: Methods and Applications”]