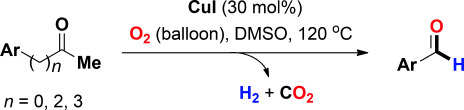Tang, C.; Jiao, N.; Angew. Chem. Int. Ed. 2014, Early view.
DOI:10.1002/anie.201403528
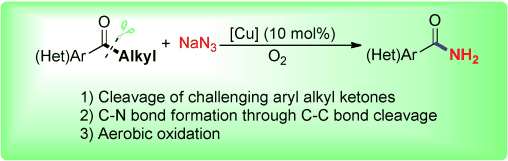
A novel copper-catalyzed aerobic oxidative C(CO)-C(alkyl) bond cleavage reaction of aryl alkyl ketones for C-N bond formation is described. A series of acetophenone derivatives as well as more challenging aryl ketones with long-chain alkyl substituents could be selectively cleaved and converted into the corresponding amides, which are frequently found in biologically active compounds and pharmaceuticals.
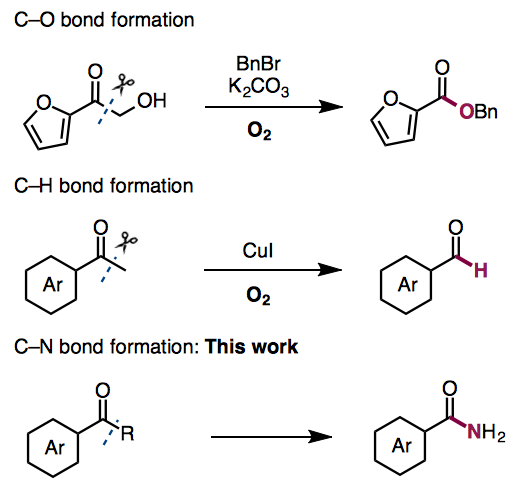
Recently, aerobic oxidative C-C bond cleavage of carbonyl compounds have been reported. Jiang and co- workers reported a transition-metal-free aerobic oxidative C-C bond cleavage of alpha-hydroxyketones and subsequent esterification.[1] The group of Bi and Liu reported a copper-catalyzed C(CO)-C(methyl) bond cleavage reaction under oxygen atmosphere, which terminates at the aldehyde stage without over oxidization.[2]
In this time, Jiao group developed a novel copper-catalyzed aerobic oxidative C-C bond cleavage reaction for C-N bond formation,which enables the direct transformation of aryl alkyl ketones into benzamides with high efficiency.
-
References
[1] “Transition-Metal-Free Aerobic Oxidative Cleavage of C-C Bonds in α-Hydroxy Ketones and Mechanistic Insight to the Reaction Pathway”
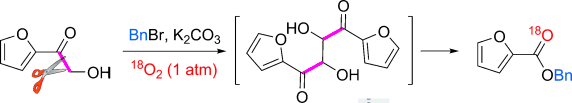
Liu, H.; Dong, C.; Zhang, Z.; Wu, P. Jiang, X. Angew. Chem. Int. Ed. 2012, 51, 12570. DOI: 10.1002/anie.201207206
Clear cut: For the title reaction, O2, the ideal oxidant, was used as the only oxidizing reagent. The dimer intermediate (see scheme) and isotopic labeling control experiments with18O2 partially disclosed the reaction mechanism.
[2] “Chemoselective Oxidative C(CO)-C(methyl) Bond Cleavage of Methyl Ketones to Aldehydes Catalyzed by CuI with Molecular Oxygen”
Zhang, L.; Bi, X.; Guan, X.; Li, X.; Lin, Q.; Barry, B.-D., Liao, P. Angew. Chem. Int. Ed. 2013, 52, 11303–11307. DOI:10.1002/anie.201305010
Aldehyde Termination: A novel copper-catalyzed transformation from methyl ketones into aldehydes has been accomplished. This method is applicable to a wide range of aromatic and aliphatic methyl ketones and chemoselectively produces aldehydes, accompanied by the release of hydrogen (H2) and carbon dioxide (CO2) as by-products.
-
Related Books
[amazonjs asin=”B00BLQYKGM” locale=”US” title=”Transition Metal Organometallic Chemistry (SpringerBriefs in Molecular Science)”][amazonjs asin=”364234285X” locale=”US” title=”Inventing Reactions (Topics in Organometallic Chemistry)”]
-
Related Links
The Ning Jiao group