- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
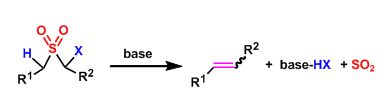
The basic treatment of α-halogenated sulfones leads to the formation of alkenes with the elimination of sulfur dioxide. This reaction is called the Ramberg-Backlund reaction and provides an indirect route to make carbon-carbon bonds.
-
General References
- Ramberg, L.; Backlund B. Arkiv Kemi, Minerat. Geol. 1940, 13A, 50.
- Paquett, L. A. Acc. Chem. Res. 1968, 1, 209. DOI: 10.1021/ar50007a003
- Paquett, L. A. Org. React. 1977, 25, 1. doi:10.1002/0471264180.or025.01
- Hartman, G. D.; Hartman, R. D. Synthesis 1982, 504.
- Clough, J. M. Comprehensive Organic Synthesis 1991, 3, 861.
- Taylor, R. J. K. Chem. Commun. 1999, 217. DOI: 10.1039/a806615i
-
Reaction Mechanism
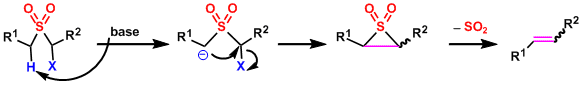
The α-protons of sulfones are reasonably acidic and easily deprotonated. The intramolecular substitution forms three-membered episulfone intermediates, which decompose to give alkenes with SO2 as a leaving group.
-
Examples
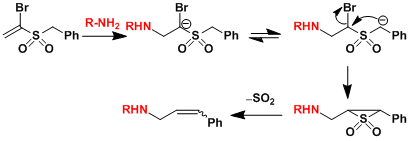
As shown below, it is possible to generate the anion via conjugate addition to vinyl sulfones and trigger the Ramberg-Backlund reaction in tandem fashion.[1]
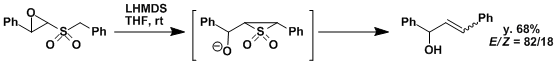
An example in which the reaction was combined with epoxide ring opening to synthesize allyl alcohols.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Burger, J. J.; Chen, T. B. R. A. ; de Waard, E. R.; Huisman, H. O. Tetrahedron 1981, 37, 417. doi:10.1016/S0040-4020(01)92030-7 [2] (a) Evans, P.; Taylor, R. J. K. Tetrahedron Lett. 1997, 38, 3055. doi:10.1016/S0040-4039(97)00507-8 (b) Evans, P.; Johnson, P.; Taylor, R. J. K. Eur. J. Org. Chem. 2006, 1740.doi: 10.1002/ejoc.200500956
-
Related Reactions
-
Related Books
-
External Links
Ramberg-Backlund reaction (Wikipedia)