- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
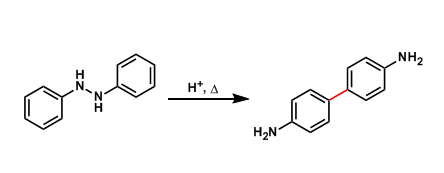
Under acidic conditions, 1,2-diphenylhydrazines are known to rearrange into 4,4’-diaminobiphenyls (benzidines).
-
General References
- Hofmann, A. W. Proc. Roy. Soc. London 1863, 12, 576.
- Jacobson, P. et al. Ber.1893, 26, 688.
-
Reaction Mechanism
The rearrangement is thought to proceed via concerted [5,5]-sigmatropic rearrangement. (Ref: J. Am. Chem. Soc. 1981, 103, 955; J. Am. Chem. Soc. 1982, 104, 2501; J. Am. Chem. Soc. 1984, 106, 7077.) 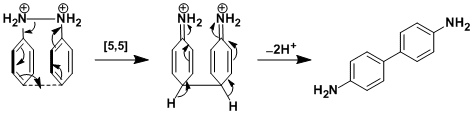
When the para position is substituted, rearrangement occurs at the ortho position ([3,3]-sigmatropic rearrangement, the diaza-Cope rearrangement).
-
Examples
In 2013, List and Kürti independently reported asymmetric benzidine rearrangement catalyzed by chiral phosphoric acids, demonstrating the synthesis of 1,1’-binaphthyl-2,2’-diamine (BINAM) in impressively high yields and enantioselectivities.[1]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] (a) Kanta, C.; Fabio, D.; Pesciaioli, F.;List, B. Angew. Chem. Int. Ed. 2013, 52, 9293. DOI: 10.1002/anie.201304039 (b) Li, G-Q.; Gao, H.; Keene, C.; Devonas M.; Ess, D.H.; Kürti, L. J. Am. Chem. Soc.. 2013, 135, 7414 DOI: 10.1021/ja401709k
-
Related Reactions
-
Related Books
[amazonjs asin=”0080966306″ locale=”US” title=”Organic Syntheses Based on Name Reactions, Third Edition: a practical guide to 750 transformations”]
-
External Links