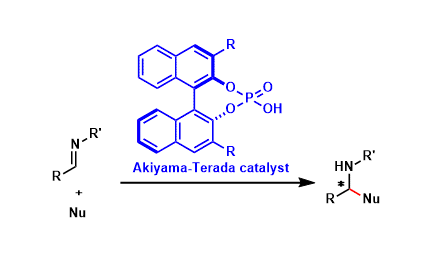
-
General Characteristics
The phosphoric acids containing an appropriately substituted BINOL backbone function as chiral Brønsted acid catalysts that can promote a wide range of stereoselective reactions.
Reported independently in 2004 by the laboratories of Takahiko Akimaya (of Gakushuin University, Japan) and Masahiro Terada (Tohoku University, Japan), these catalysts are called the Akiyama-Terada catalysts.
A number of modified catalysts have been reported more recently, including the imide version and the chiral counter anion version. The versatility of these catalysts makes them one of the most popular organocatalyst classes known today.
-
General References
- Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Angew. Chem. Int. Ed. 2004, 43, 1566. DOI: 10.1002/anie.200353240
- Uraguchi, D.; Terada, M. J. Am. Chem. Soc.2004, 126, 5365. DOI: 10.1021/ja0491533
<reviews>
- Akiyama, T.; Itoh, J.; Fuchibe, K. Adv. Synth. Catal. 2006, 348, 999. DOI: 10.1002/adsc.200606074
- Akiyama, T. Chem. Rev.2007, 107, 5744. DOI: 10.1021/cr068374j
- Doyle, A. G.; Jacobsen, E. N. Chem. Rev.2007, 107, 5713. DOI: 10.1021/cr068373r
- Terada, M. Chem. Commun.2008, 4097. DOI: 10.1039/B807577H
- Adair, G.; Mukherjee, S.; List, B. Aldrichimica Acta2008, 41, 31. [website]
- Zamfir, A.; Schenker, S.; Freund, M.; Tsogoeva, S. B. Org. Biomol. Chem.2010, 8, 5262. DOI: 10.1039/C0OB00209G
- Terada, M. Bull. Chem. Soc. Jpn.2010, 83, 101. doi:10.1246/bcsj.20090268
- Terada, M. Synthesis2010, 1929. DOI: 10.1055/s-0029-1218801
- Rueping, M.; Kuenkel, A.; Atodiresei, I. Chem. Soc. Rev.2011, 40, 4539. DOI: 10.1039/C1CS15087A
- Akiyama, T. J. Synth. Org. Chem. Jpn. 2011, 69, 913. doi:10.5059/yukigoseikyokaishi.69.913
- Lv, F.; Liu, S.; Hu, W. Asian J. Org. Chem.2013, 2, 824. DOI: 10.1002/ajoc.201300097
<reviews for chiral counteranion catalysis>
- Lacour, J.; Moraleda, D. Chem. Commun. 2009, 7073. DOI: 10.1039/b912530b
- Phipps, R. J.; Hamilton, G. L.; Toste, F. D. Nat. Chem.2012, 4, 603. doi:10.1038/nchem.1405
- Mahlau, M.; List, B. Angew. Chem. Int. Ed.2013, 52, 518. DOI: 10.1002/anie.201205343
-
Reaction Mechanism
-
Examples
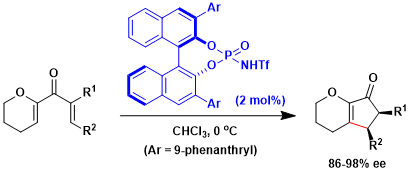
The phosphoric acid-catalyzed reactions are by no means limited to additions to imines. An example shown below is the asymmetric Nazarov cyclization.[1]
In the asymmetric α-allylation of aldehydes via π-allylpalladium, the phosphoric acid and the palladium can coexist and cooperate to achieve the synthesis of all-carbon quaternary stereogenic centers in high enantioselectivity.[2] 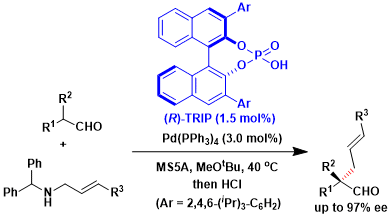
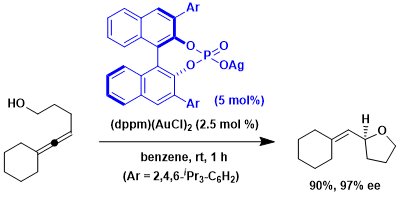
An example in which the phosphoric acid was used as the counter anion of a gold catalyst.[3] This concept is helpful when neutral chiral ligands are ineffective in creating the chiral environment.
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Rueping, M.; Ieawsuwan, W.; Antonchick, A. P.; Nachtsheim, B. J. Angew. Chem. Int. Ed. 2007, 46,
2097. DOI: 10.1002/anie.200604809 [2] Mukherjee, S.; List, B. J. Am. Chem. Soc. 2007, 129, 11336. DOI: 10.1021/ja074678r [3] Hamilton, G. L.; Kang, E. J.; Mba, M.; Toste, F. D. Science 2007, 317, 496. DOI:10.1126/science.1145229
-
Related Reactions
-
Related Books
[amazonjs asin=”B00BLRNHYC” locale=”US” title=”Development of Novel Hydrogen-Bond Donor Catalysts (Springer Theses)”]
[amazonjs asin=”3527332367″ locale=”US” title=”Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, 3 Volume Set”]
[amazonjs asin=”3709111625″ locale=”US” title=”Asymmetric Organocatalysis in Natural Product Syntheses (Progress in the Chemistry of Organic Natural Products)”]
-
External Links
- 東北大学 寺田眞浩 研究室 (Tohoku University Terada Laboratory)
- 学習院大学 秋山隆彦 研究室 (Gakushuin University Akiyama Laboratory)