- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
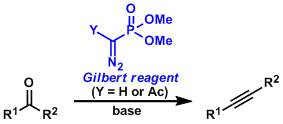
Aldehydes and ketones can be converted into alkynes with one carbon homologation using the α-diazophosphonate compound called the Gilbert reagent.
When Y=H, the reaction needs a strong base such as tBuOK to proceed and base sensitive reactants are inevitably incompatible. However, with the modified reagent in which Y=Ac, milder bases such as potassium carbonate can be used, so the substrate scope is wider (the Ohira-Bestmann modification).
-
General References
・Seyferth, D.; Hilbert, P.; Marmor, R. S. J. Am. Chem. Soc. 1967, 89, 4811; J. Org. Chem. 1971, 36, 1379. doi:10.1021/jo00809a014
・Gilbert, J. C.; Weerasooriya, U. J. Org. Chem. 1979, 44, 4997; ibid. 1982, 47, 1837. doi:10.1021/jo00349a007
<Ohira-Bestmann Modification>
・Ohira, S. Synth. Commun. 1989, 19, 561.
・Müller, S.; Liepold, B.; Roth, G. J.; Bestmann, H. J. Synlett 1996, 521. doi:10.1055/s-1996-5474
・Roth, G. J.; Liepold, B.; Muller, S. G.; Bestmann, H. J. Synthesis 2004, 59. doi:10.1055/s-2003-44346
<Preparation>
・Callant, P.; D’haenens, L.; Vandewalle, M. Synth. Commun. 1984, 14, 155.
・Brown, D. G.; Velthuisen, E. J.; Commerford, J. R.; Brisbois, R. G.; Hoye, T. R. J. Org. Chem. 1996, 61, 2540. doi:10.1021/jo951944n
-
Reaction Mechanism
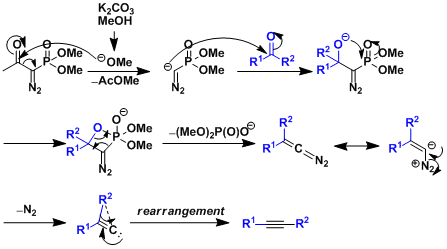
-
Examples
Lithiated TMS diazomethane also works for the same transformation.[1] Shown below is an example where it is used in the synthesis of (+)-Ambruticin.[2]
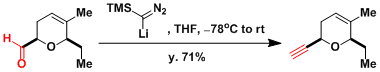
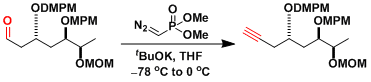
An example in the context of bryostatin synthesis.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Miwa, K.; Aoyama, T.; Shioiri, T. Synlett 1994, 107.
[2] Liu, P.; Jacobsen, E. N. J. Am. Chem. Soc. 2001, 123, 10772. DOI: 10.1021/ja016893s
[3] De Brabander, J.; Vandewalle, M. Synthesis 1994, 855.
-
Related Books
[amazonjs asin=”352730634X” locale=”US” title=”Modern Carbonyl Olefination: Methods and Applications”]