How many of you are aware with Moore’s law?
This law states that the number of transistors in integrated circuits doubles about every two years. It has been said that this increment is gradually reaching a limit, but a recent report sheds light on this premonition.
Associate Professor Hayato Tsuji and co-workers of Professor Eiichi Nakamura’s group at Tokyo University have discovered that the rate of electron transfer through molecular wires can be increased by 840-fold at room temperature when using π-conjugated molecular wires as bridges with respect to flexible equivalents.
This article reports the first example of a molecular bridge operating at room temperature.
“Electron transfer through rigid organic molecular wires enhanced by electronic and electron-vibrating coupling”
Sukegawa, J.; Schubert, C.; Zhu, X.; Tsuji, H.; Guldi, D.M.; Nakamura, E. Nat. Chem. 2014, 6, 899-905.
DOI: 10.138/nchem.2026
Improving function through design at the molecular level
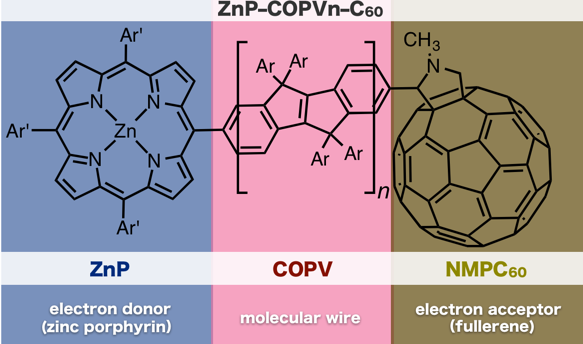
Tsuji and his colleagues have developed a carbon-bridged oligo-p-phenylenevinylene (COPV) for use as a molecular bridge.
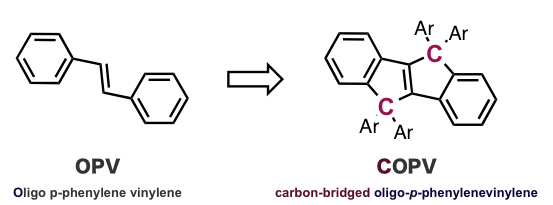
COPV exhibits a flat structure due to bridged carbons (showing monomer unit)
Oligo-p-phenylenevinylene (OPV), a molecular wire used in previous studies, is unable to take up a flat structure due to proximal hydrogens. COPV was designed through fixing the flexible portions with carbon atoms, enabling conjugation of π electrons.
The figure above shows the molecules used in this study. The rate of electron transfer between zinc porphyrin (electron donor) and fullerene (electron acceptor) connected by COPV was measured. In comparison to the OPV-bridged system, the rate of electron transfer in the COPV-bridged system was increased by 6.5 and 840 times during charge separation and charge recombination, respectively.
The key to this discovery arises from the flat structure of the π-conjugated COPV molecule.
Design and control of the structure on the molecular level
The main significance of this study was the identification of a constructable organic molecule that can be used as a molecular wire in nanodevices at room temperature.
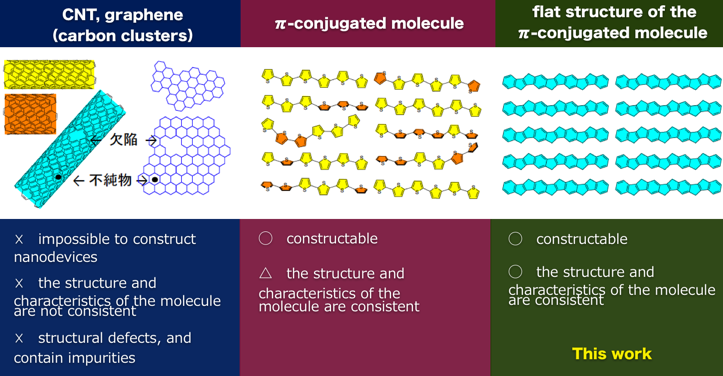
The phenomena reported in this research has been observed in inorganic semiconductors such as quantum dots, carbon clusters such a carbon nanotubes and organic molecules such as quinones. However, in organic molecular wires this phenomena could only be observed in molecules fixed on a plate at -270 °C.
In order to function as a nanodevice, it is essential for the molecules to operate at room temperature. In addition, it is also important that the structure and characteristics of the molecule are consistent throughout the device.
For example, although oligothiophenes are constructable as molecular wires, the position of thiophene units differs within each molecule.
When it comes to carbon clusters, it becomes impossible to construct nanodevices, as the polymer-like carbon nanotubes and graphenes vary in molecular weight, have structural defects, and contain impurities.
On the other hand, with these COPV-containing systems, they exhibit a singular structure, which may contribute towards the development of uniform devices and molecular computers.
e-v coupling
The molecules reported in this research show e-v coupling, which is an interaction where the energy lost during electron transfer causes nuclear vibration. Molecules with a uniform structure exhibiting this coupling have physicochemical significance.
According to the Born-Oppenheimer approximation and the Franck Condon principle, electrons move faster than the nucleus during an electronic transition. However, the COPV system does not obey these traditional principles. This was actually the first time that the breakdown of these principles was observed in solution phase at room temperature.
Being able to measure the uniform structure in solution phase suggests that it is possible to evaluate structural-physical property relationships. This is important from the perspective of fundamental physical chemistry.
If research on molecular wires evolves from this discovery and becomes applicable, this may surpass Moore’s law and lead to further miniaturisation of integrated circuits.
Message from Dr. Jyunpei Sukegawa
Dr. Junpei Sukegawa, the first author of this study speaks about his experience regarding his work.
Looking back at this research, I believe that this is a result of our long-term research efforts. The main points that led to the outcome of this research can be summarised as follows:
- Development of COPV, which has a flat structure highly desirable for extended π-conjugation.
- Prof. Guldi’s (a good friend of Prof. Nakamura) research on long distance electron transfer through molecular wires.
- Persistence to clarify unexpected research results.
The turning point of this research was when Prof. Guldi presented his research on electron transfer of OPV during a seminar at our university. At the time, OPV was known to be the best molecular wire. Considering the structure of COPV that we were developing at that time, we expected it to function as an effective molecular wire and decided to start a collaboration with Prof. Guldi. We finished the synthesis of the molecule after about a year and a half and Prof. Guldi measured the transient absorption of the molecule. As expected, the rate of electron transfer was enhanced but there were some observations that were difficult to explain.
- There was no consistency in the dependence of the rate of electron transfer on solution and distance.
- Large rearrangement energy arising from structural change despite the molecule having a rigid framework.
- Relatively fast charge recombination compared to charge separation (As stated in (2), we expected the rearrangement energy to be small and thus the charge recombination to be slow).
After half a year of evaluation, we were able to logically explain (1), but a further one and a half year passed without finding a reasoning for (2) and (3). Meanwhile, I was working on another theme but when I presented a poster at a symposium on this topic, the problems associated with (2) and (3) were pointed out. On this opportunity, I started to study electron transfer theory from scratch to explain the above issues, and considered using Marcus theory that takes molecular vibration into account . Upon analysis of the results with regard to molecular vibration, it was possible to explain both problems by e-v coupling. However, a new question arose as to why e-v coupling became prominent in this particular molecule. Going through literature on solid-state physics to see whether there were any electron transfer mechanisms that couple electron transfer and molecular vibration, I finally found a phenomenon called inelastic tunneling. In flexible molecular wires such as OPV, this effect does not appear unless at very low temperature conditions where molecular motion is frozen. On the other hand, this phenomenon was observed in the rigid COPV even at room temperature. Since this was the first example of e-v coupling on an intermolecular charge transfer system at room temperature, we decided to submit this research to Nature Chemistry. Currently, we are working with theoreticians to elucidate the origin of e-v coupling.
Finally, I would like to summarise that this research is a result of interdisciplinary collaboration, originating from synthesis of a novel molecule. In addition, I believe that this unique result arises from looking closely at the experimental data and persistence to finish the work. The process of understanding unknown matters was both tough and enjoyable, and I was able to feel that my 4-year efforts really paid off when this research was accepted for publication. I would like to express my respect and gratitude towards Professors Nakamura, Tsuji, and Guldi as well as the senior researchers who carried out the fundamental research of this work.