- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The Clemmensen reduction is the reductive conversion of carbonyl groups into corresponding methylene units using zinc amalgam-HCl, which works particularly well for the reduction of aryl ketones.
Alkyl ketones can be reduced under anhydrous conditions using a solution of HCl in ether. In this case, metallic zinc can be used in place of the toxic mercury-containing reagents.
Electrolytic conditions are also known as an alternative to using metal reagents.
The reaction conditions are highly acidic, therefore this reaction is used complementarily with the Wolff-Kishner reduction (which is done under basic conditions) and thioacetal desufurization (which is mediated by Raney nickel).
-
General References
・ Clemmensen, E. Ber. 1913, 46, 1837.
・ Clemmensen, E. Ber. 1914, 47, 51.
・ Clemmensen, E. Ber. 1914, 47, 681.
・ Martin, E. L. Org. React. 1942, 1, 155.
・ Nakabayashi T. J. Am. Chem. Soc. 1960, 82, 3900. DOI: 10.1021/ja01500a029
・ Buchanan, J. G. St. C.; Woodgate, P. D. Quart. Rev. 1969, 23, 522.
・ Vedejs, E. Org. React. 1975, 22, 401.
・ Yamamura, S.; Nishiyama, S. Comprehensive Organic Synthesis 1991, 8, 309.
-
Reaction Mechanism
The reaction proceeds on the surface of zinc amalgam. The exact mechanism is unknown but the one shown below is a reasonable explanation.
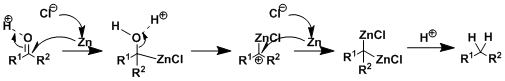
-
Examples

-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books

