- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
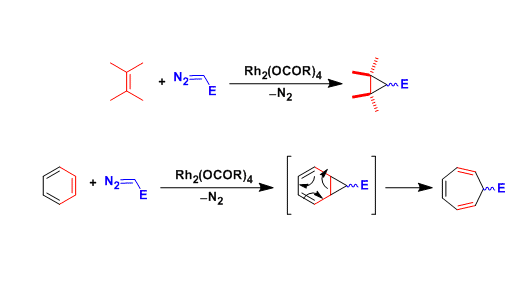
In the presence of metal catalysts, diazo compounds decompose to generate metal carbenoid species, which react with olefins to give cyclopropanes.
Metal carbenoids also react with the double bond of aromatic rings. In this case, the rearrangement of divinylcyclopropane leads to the formation of cycloheptatriene skeleton (the Buchner ring expansion).
With an appropriate choice of chiral ligands, asymmetric catalysis is possible.
-
General References
<Buchner ring expansion>
- Buchner, E. Ber. 1896, 29, 106. (1896);
- Buchner, E.; Schottenhammer, K. Ber.1920, 53, 865.
- Anciaux, A. J.; Demonceau, A.; Noels, A. F.; Hubert, A. J.; Warin, E.; Teyssie. P. J. Org. Chem.1981, 46, 873. DOI: 10.1021/jo00318a010
- Review: Ye, T.; McKervey, M. A. Chem. Rev.1994,94, 1091. DOI: 10.1021/cr00028a010
-
Reaction Mechanism
Please see the page titled “C-H Insertion of Metal Carbenoids.”
-
Examples
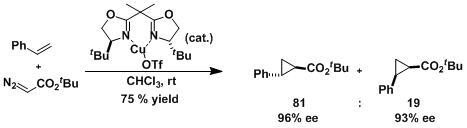
An example of intermolecular asymmetric cyclopropanation.[1]
The synthesis of a 7-membered ring from a phenyl ring in the total synthesis of harringtonolide.[2]
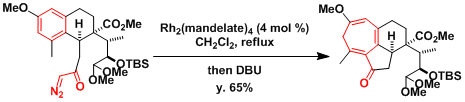
The copper-catalyzed cyclopropanation was used in the synthesis of (-)-verbenalol and (-)-epiverbenalol.[3]

The generic reaction mechanism is as follows.

There are impressively many intermolecular examples.[4]

The sequence of cyclopropanation and the Cope rearrangement is a great way of synthesizing 7-membered rings, including this lactone compound shown here.[5]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] Evans, D. A. et al. J. Am. Chem. Soc. 1991, 113, 726. DOI: 10.1021/ja00002a080 [2] (a) Mander, L. N. et al. J. Am. Chem. Soc. 1998, 120, 1914. DOI: 10.1021/ja9738081 (b) Mander, L. N. et al. Aust. J. Chem. 2000, 53, 819. doi:10.1071/CH00124 [3] Laabassi, M.; Grbe, R. Tetrahedron Lett. 1988, 29, 611. DOI: 10.1016/S0040-4039(00)80163-X [4] Marino, J. P.; Silveira, C.; Comasseto, J.; Petragnani, N. J. Org.Chem. 1987, 52, 4139. DOI: 10.1021/jo00227a042 [5] Davies, H. M. L.; McAfee, M. J.; Oldenburg, C. E. M. J. Org. Chem. 1989, 54, 930. DOI: 10.1021/jo00265a037
-
Related Reactions
- diazomethane
- Regitz Diazo Transfer
- Vinylcyclopropane Rearrangement
- Kulinkovich Reaction
- Du Bois Amination
- C-H Insertion of Metal Carbenoid
- Corey-Chaykovsky Reaction
- Simmons-Smith Reaction
-
Related Books
[amazonjs asin=”3639182804″ locale=”US” title=”Intramolecular Cyclopropanation/Thermal Fragmentation strategy: Intramolecular Reactions of Diazo Compounds in Natural Product Synthesis”]