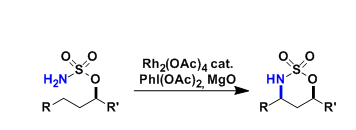- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The direct amination of unactivated C-H bonds utilizing metal nitrenoids, especially with Rh-catalysis, became a practical synthetic option in the early 2000’s. This reaction has been used in a number of alkaloid syntheses and is an example that reflects the current trend of organic synthesis where C-H activation is becoming more and more mainstream.
-
General References
- Espino, C. G.; Du Bois, J.. Angew. Chem. Int. Ed. 2001, 40, 598. [abstract]
- Espino, C. G.; Wehn, P. M.; Chow, J.; Du Bois, J. J. Am. Chem. Soc. 2001, 123, 6935.DOI: 10.1021/ja011033x
- Fiori, K. W.; Fleming, J. J.; Du Bois, J. Angew. Chem. Int. Ed. 2004, 43, 4349. doi:10.1002/anie.200460791
- Fiori, K. W.; Du Bois, J. J. Am. Chem. Soc. 2007, 129, 562. DOI: 10.1021/ja0650450
- Davies, H. M. L.; Manning, J. R. Nature 2008, 451, 417. DOI:10.1038/nature06485
-
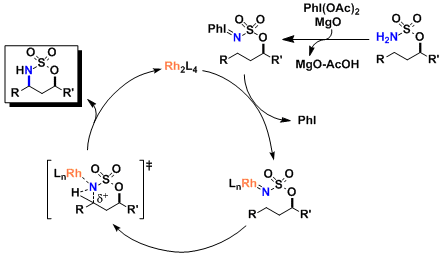
Reaction Mechanism
Magnesium oxide is added to trap acetic acid, which is formed with the formation of the iodoimine and known to lower the catalytic activity.
The active species is the rhodium nitrenoid.
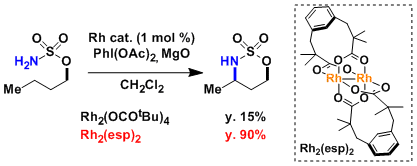
As can be seen in the example shown below, it is important that the rhodium catalyst retains a dinuclear structure. (Ref: Nakamura, E. et al. J. Am. Chem. Soc. 2002, 124, 7181.)
The reaction goes through a cationic intermediate, therefore substrates that are capable of stabilizing the positive charge (positions adjacent to heteroatoms, tertiary carbon, etc.) are more reactive.
-
Examples
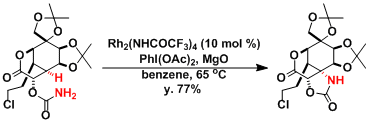
The Du Bois lab demonstrated that the reaction can be used in the synthesis of extremely complex natural products, such as tetrodotoxin.[1]
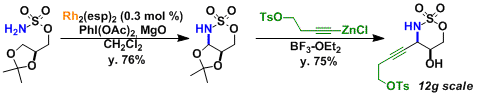
The synthesis of saxitoxin[2]: The intermediate containing contiguous stereogenic centers was prepared by stereoselective alkynylation of the aminal synthesized by C-H amination.[3]
The ligands that form a stable dinuclear structure with rhodium, such as esp, are known to increase catalytic activity and expand substrate scope.[4]
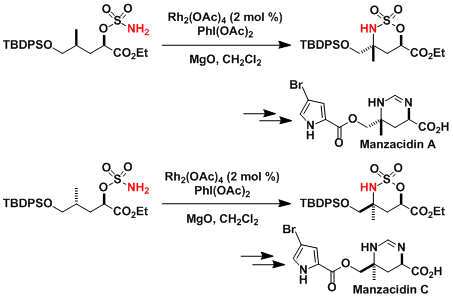
The stereoselectivity of C-H amination was applied perfectly to the total synthesis of manzacidins.[5]
Total synthesis of (+)-gonyautoxin 3[6]: The same catalytic system was effectively used for the amination of the pyrrole.[7]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] Hinman, A.; Du Bois, J. J. Am. Chem. Soc. 2003, 125, 11510. DOI: 10.1021/ja0368305 [2] (a) Fleming, J. J.; Du Bois, J. J. Am. Chem. Soc. 2006, 128, 3926. DOI: 10.1021/ja0608545 (b) Fleming, J. J.; McReynolds, M. D.; Du Bois, J. J. Am. Chem. Soc. 2007, 129, 9964. DOI: 10.1021/ja071501o [3] (a) Fleming, J. J.; Fiori, K. W.; Du Bois, J. J. Am. Chem. Soc. 2003, 125, 2028. DOI: 10.1021/ja028916o (b) Fiori, K. W.; Fleming, J. J.; Du Bois, J. Angew. Chem. Int. Ed. 2004, 43, 4349. doi:10.1002/anie.200460791 [4] Espino, C. G.; Fiori, K. W.; Kim, M.; Du Bois, J. J. Am. Chem. Soc. 2004, 126, 15378. DOI: 10.1021/ja0446294 [5] Wehn, P. M.; Du Bois, J. J. Am. Chem. Soc. 2002, 124, 12950. DOI: 10.1021/ja028139s [6] Mulcahy, J. V.; Du Bois, J. J. Am. Chem. Soc. 2008, 130, 12630. doi:10.1021/ja805651g [7] Kim, M.; Mulcahy, J. V.; Espino, C. G.; Du Bois, J. Org. Lett. 2006, 8, 1073. DOI: 10.1021/ol052920y
-
Related Reactions
- Ichikawa Allylcyanate Rearrangement
- Cross Dehydrogenative Coupling (CDC)
- Catalytic C-H Oxidation
- Hofmann-Löffler-Freytag Reaction
- Catalytic C-H activation
- Cyclopropanation with Metal Carbenoid
- Overman Rearrangement
- C-H Insertion of Metal Carbenoid
- Curtius Rearrangement
-
Related Books
[amazonjs asin=”3527306838″ locale=”US” title=”Modern Rhodium-Catalyzed Organic Reactions”]
-
External Links
Justin Du Bois (Stanford Univ.)