- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The Pinnick (or Kraus) oxidation reaction converts aldehydes into carboxylic acids under especially mild conditions.
The Jones oxidation, PDC, ruthenium tetroxide, etc. are all capable of the one-step oxidation of alcohols to carboxylic acids, but their reaction conditions tend to be too harsh to be used for polyfunctionalized compounds in the late stage of synthesis. Milder and stepwise oxidation methods are often used instead. The Pinnick oxidation is the second step of the transformation.
-
General References
・Kraus, G. A.; Roth, B. J. Org. Chem. 1980, 45, 4825. DOI: 10.1021/jo01312a004
・Kraus, G. A.; Taschner, M. J. J. Org. Chem. 1980, 45, 1175. DOI: 10.1021/jo01294a058
・Bal, B. S.; Childers, W. E., Jr.; Pinnick, H. W. Tetrahedron 1981, 37, 2091. doi:10.1016/S0040-4020(01)97963-3
-
Reaction Mechanism
2-Methyl-2-butene works as the scavenger of byproducts such as hypochlorous acid and chlorine to prevent undesired side reactions.
-
Examples
The most salient characteristics of this reaction is the high functional group tolerance.
An application in the total synthesis of norzoanthamine.[1]
Another example in the synthesis of platensimycin.[2]![pinnick_4[1]](https://assets.en.chem-station.com/uploads/2014/05/pinnick_41.gif)
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Miyashita, M.; Tanino, K. et al. Science 2004, 305, 495. DOI: 10.1126/science.1098851
[2] (a) Nicolaou, K. C.; Li, A.; Edmonds, E. J. Angew. Chem. Int. Ed. 2006, 45, 7086. doi:10.1002/anie.200603892 (b) Nicoalou, K. C.; Edmonds, E. J.; Li, A.; Tria, G. S. Angew. Chem. Int. Ed. 2007, 46, 3942. doi:10.1002/anie.200700586
-
Related Books

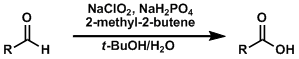
![pinnick_2[1]](https://assets.en.chem-station.com/uploads/2014/05/pinnick_21.gif)
![pinnick_3[1]](https://assets.en.chem-station.com/uploads/2014/05/pinnick_31.gif)