- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Sulfonyl groups are stable under both acidic and basic conditions. They are also used for purposes other than protection.
Commonly used groups are methanesulfonyl (Ms), toluenesulfonyl (Ts), nitrobenzenesulfonyl (Ns), and trifluoromethanesulfonyl (Tf).
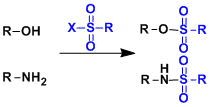
Sulfonylation of hydroxyl groups makes them more reactive as a leaving group in substitution and elimination reactions, thus sulfonyl protection is rarely used for aliphatic alcohols. On the other hand, it is often used to protect electron-rich phenols against oxidation due to its electron withdrawing property.
Sulfonyl groups are effective in protecting amines. The nucleophilicity and basicity of amines can be reduced by protecting them as sulfonamides.
Sulfonamides of primary amines have a sufficiently acidic proton, which can be used to synthesize secondary amines under the Mitsunobu or other alkylation conditions. For this purpose, more easily removable Ns group is used more frequently than Ts group (the Fukuyama amine synthesis).
-
General References
-
Reaction Mechanism
The protections of Ms and Ts groups proceed through different mechanisms. Different bases are used in the two reactions to maximize the formation of reactive species. In general, triethylamine is used for Ms protection, while pyridine (or DMAP/triethylamine) is used for Ts protection.
The deprotection of sulfonyl groups is relatively difficult. When it has to be done under mild conditions, one-electron reductive conditions (e.g. Mg/MeOH) can be used.
Ns group is synthetically valuable as it can be deprotected by nucleophilic addition of a thiolate (the Fukuyama amine synthesis).
-
Examples
Tf2O is a common triflation reagent but often doesn’t work as well for the preparation of enol triflates. As alternatives, the McMurry’s and the Comin’s reagents are used.
Deprotection of Ts group by Mg-MeOH.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Sridhar, M.; Ashokkumar, B.; Narendar, R. Tetrahedron Lett. 1998, 39, 2847. doi:10.1016/S0040-4039(98)00314-1
-
Related Books
[amazonjs asin=”0471697540″ locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”]
[amazonjs asin=”B008AUBOZ2″ locale=”US” title=”Protecting Group Chemistry (00) by Robertson, Jeremy Paperback (2000)”]

![PG_sulfonyl_2[1]](https://assets.en.chem-station.com/uploads/2014/05/PG_sulfonyl_21.gif)
![PG_sulfonyl_3[1]](https://assets.en.chem-station.com/uploads/2014/05/PG_sulfonyl_31.gif)
![PG_sulfonyl_4[1]](https://assets.en.chem-station.com/uploads/2014/05/PG_sulfonyl_41.gif)
![PG_sulfonyl_5[1]](https://assets.en.chem-station.com/uploads/2014/05/PG_sulfonyl_51.gif)
![PG_sulfonyl_7[1]](https://assets.en.chem-station.com/uploads/2014/05/PG_sulfonyl_71.gif)