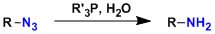- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
In the Staudinger reaction, azide compounds react with phosphines to form iminophosphorane species, which can be hydrolyzed to give primary amines. The iminophosphorane also serves as a versatile intermediate in various reactions such as the aza-Wittig reaction, in which it reacts with carbonyl compounds to give imines.
The Staudinger reaction is useful when hydrogenation cannot be used.
-
General References
・Staudinger, H.; Meyer, J. Helv. Chim. Acta 1919, 2, 635. DOI: 10.1002/hlca.19190020164
・Gololobov, Y. G.; Zhmurova, I. N.; Kasukhin, L. F. Tetrahedron 1981, 37, 437.doi:10.1016/S0040-4020(01)92417-2
・Scriven, E. F. V.; Turnbull, K. Chem. Rev. 1988, 88, 297. DOI: 10.1021/cr00084a001
・Gololobov, Y. G.; Kasukhin, L. F. Tetrahedron 1992, 48, 1353. doi:10.1016/S0040-4020(01)92229-X
・Review: Shah, S.;Protasiewicz,J. D. Coord. Chem. Rev. 2000, 210, 181. doi:10.1016/S0010-8545(00)00311-8
・Kohn, M.; Breinbauer, R. Angew. Chem. Int. Ed. 2004, 43, 3106. DOI: 10.1002/anie.200401744
-
Reaction Mechanism
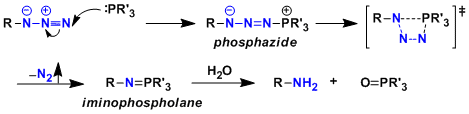
Reference: J. Org. Chem. 2004, 69 , 4299. J. Am. Chem. Soc. 2005, 127, 2686.
-
Examples
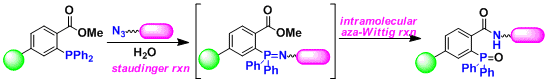
The Staudinger-Bertozzi ligation[1]: Bertozzi developed the Staudinger-then-aza-Wittig reaction shown below and demonstrated its utility in ligating large phosphorescent compounds by the amide bond. This reaction is high yielding and highly chemoselective, offering possibilities for applications in various chemical biology researches.
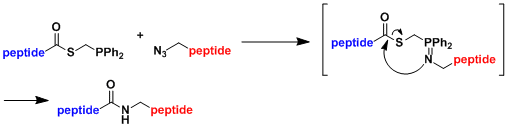
The “traceless” Staudinger ligation[2]: Cystein is not required as it was in traditional native chemical ligation.
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Saxon, E.; Bertozzi, C.R. Science 2000, 287, 2007. doi: 10.1126/science.287.5460.2007
[2] (a) Saxon, E.; Armstrong, C.R.; Bertozzi, C.R. Org. Lett. 2000, 2, 2141. DOI: 10.1021/ol006054v (b) Nilsson, B.L.; Kiessling, L.L.; Raines, R.T. Org. Lett. 2000, 2, 1939. DOI: 10.1021/ol0060174
-
Related Books