- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
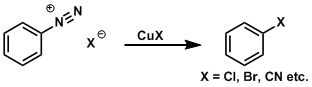
The transformation of aromatic diazonium compounds is known as the Sandmeyer reaction. Diazonium compounds are readily prepared from precursors such as nitro and aniline compounds.
-
General References
・Sandmeyer, T. Ber. 1884, 17, 1633.
・Sandmeyer, T. Ber. 1884, 17, 2650.
・Hodgson, H. H. Chem. Rev. 1947, 40, 251. DOI: 10.1021/cr60126a003
・Galli, C. Chem. Rev. 1988, 88, 765. DOI: 10.1021/cr00087a004
-
Reaction Mechanism
The reaction is formally an aromatic nucleophilic substitution. However, the actual mechanism is thought to involve single electron transfer (SET) and not completely elucidated.
-
Examples
The reaction with CuCN gives corresponding cyano derivatives and the reaction with CuCl and CuBr gives corresponding halogenated derivatives. The reaction with sodium iodide gives iodinated derivatives, and heating diazonium tetrafluoroborate salts (which are prepared by anion exchange using silver tetrafluoroborate) gives fluorinated derivatives (the Balz-Schiemann reaction). Additionally, heating diazonium salts in water or alcohol gives corresponding phenols and aryl ethers, respectively. The diazo group can also be replaced by hydrogen by treating it with hypophosphorous acid.
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books

![sandmeyer_3[1]](https://assets.en.chem-station.com/uploads/2014/05/sandmeyer_31.gif)
![sandmeyer_2[1]](https://assets.en.chem-station.com/uploads/2014/05/sandmeyer_21.gif)