- Reagent Availability
- Experimental Ease
- Generality
- Criteria #4
- Criteria #5
-
General Characteristics
-The oxidation of alcohols based on the dimethylsulfoxide (DMSO)-oxalyl chloride system is called the Swern oxidation.
-DCC (Pfitzner-Moffatt), TFAA (modified Swern), acetic anhydride (Albright-Goldmann), and SO3-pyridine (Parikh-Doering) are alternative reagents used to activate DMSO, but oxalyl chloride is a good choice in terms of causing less side reactions. The TFAA conditions are very reactive but side reactions tend to compete more.
-The reaction is used for large scale synthesis of aldehydes because the byproducts derived from the reagents are low boiling and easy to remove. However, one should be aware of stoichiometric formation of the foul smelling dimethyl sulfide as the byproduct, which must be handled properly.
-
General References
・Huang, S.-L.; Omura, K.; Swern, D. J. Org. Chem. 1976, 41, 3329. DOI: 10.1021/jo00882a030
・Mancuso, A. J.; Huang, S.-L.; Swern, D. J. Org. Chem. 1978, 43, 2489. DOI:10.1021/jo00406a041
・Omura, K.; Swern, D. Tetrahedron 1978, 34, 1651. DOI:10.1016/0040-4020(78)80197-5
・Review: Mancuso, A. J.; Swern, D. Synthesis 1981, 165. DOI:10.1055/s-1981-29377
・Marx, M.; Tidwell, T. T. J. Org. Chem. 1984, 49, 788. DOI: 10.1021/jo00179a009
・Review: Tidwell, T. T. Org. React. 1990, 39, 297.
・Review: Tidwell, T. T. Synthesis 1990, 857. DOI:10.1055/s-1990-27036
・Review: Lee, T. V. Comprehensive Organic Synthesis 1991, 7, 291.
-
Reaction Mechanism
DMSO and oxalyl chloride react to form a chlorosulfonium salt, which acts as the active oxidant. This active species is unstable towards water and decomposes quickly above -60˚C. The reaction therefore needs to be carried out under dry and cryogenic (-78℃) conditions. The reaction also generates a toxic gas (CO) and a foul odorant (Me2S), thus should be carried out under ventilated fume hoods.
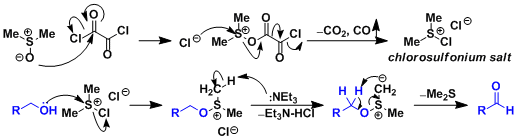
The most well-known side reaction is methylthiomethyl (MTM) etherification. At higher temperatures, the chlorosulfonium salt undergoes the Pummerer rearrangement followed by substitution by the alcohol to give a MTM-protected alcohol.
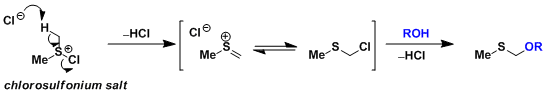
-
Examples
The reaction proceeds under mild conditions and can be used to synthesize relatively unstable aldehydes. The Swern reaction is one of the most extensively used methods among a myriad of oxidation methods and has applications in virtually every situation. An example is shown below (the synthesis of (+)-thiazinotrienomycin E).[1]

An example of TFAA conditions.[2]

-
Experimental Procedure
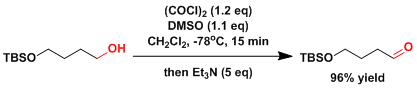
ADD EXPERIMENTAL LATER
-
Experimental Tips
-The reaction solvent is usually dichloromethane. THF and diethylether are also viable.
-Unless special precision is required, the standard molar ratio of the substrate:oxalyl chloride:DMSO:triethylamine is 1:2:3:6.
-All steps of the reaction should be carried out in a fume hood to contain the odor of dimethyl sulfide. The glassware can be deodorized by soaking them in a hypochlorite solution (bleach).
-
References
[1] Smith, A. B., III; Wan, Z. J. J. Org. Chem. 2000, 65, 3738. DOI: 10.1021/jo991958j
[2] Braish, T. F.; Saddler, J. C.; Fuchs, P. L. J. Org. Chem. 1988, 53, 3647. DOI: 10.1021/jo00251a001
[3] Taillier, C.; Gille, B.; Bellosta, V.; Cossy, J. J. Org. Chem. 2005, 70, 2097. DOI: 10.1021/jo048115z
-
Related Books
[amazonjs asin=”0387236074″ locale=”US” title=”Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice (Basic Reactions in Organic Synthesis)”][amazonjs asin=”0198556640″ locale=”US” title=”Oxidation and Reduction in Organic Synthesis (Oxford Chemistry Primers, 6)”][amazonjs asin=”3527306420″ locale=”US” title=”Modern Oxidation Methods”]

