- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-While cross coupling reactions based on organolithium and Grignard reagents (Kumada-Tamao-Corriu cross coupling) tend to suffer from unwanted side reactions when applied to complex systems due to the naturally high reactivity of the reagents, the reaction using organozinc reagents (formed by transmetallation with zinc chloride) enables cross couplings under milder conditions.
-The reactivity is generally high.
-Alkyl(sp3)zinc compounds are viable substrates.
-The reaction has high functional group tolerance.
-The reactions based on organoaluminum and organozirconium reagents are also considered as variants of Negishi coupling.
-
General References
・King, A. O.; Okukado, N.; Negishi, E. J. Chem. Soc., Chem. Commun. 1977, 683. DOI: 10.1039/C39770000683
・Negishi, E; King, A. O.; Okukado, N. J. Org. Chem. 1977, 42, 1821. DOI: 10.1021/jo00430a041
・Negishi, E. Acc. Chem. Res. 1982, 15, 340. DOI: 10.1021/ar00083a001
・Erdik, E. Tetrahedron 1992, 48, 9577. doi:10.1016/S0040-4020(01)81181-9
・Knochel, P.; Singer, R. D. Chem. Rev. 1993, 93, 2117. DOI: 10.1021/cr00022a008
・Negishi, E. et al. Aldrichimica Acta 2005, 38, 71. [PDF]
・Pd-Catalyzed Cross Coupling in Total Synthesis: Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem. Int. Ed. 2005, 44, 4442. doi:10.1002/anie.200500368
-
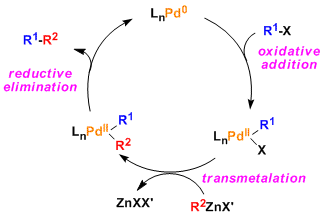
Reaction Mechanism
The basic generic catalytic cycle is same as other palladium-catalyzed cross coupling reactions. (Ref: J. Am. Chem. Soc. 2007, 129, 3508.)
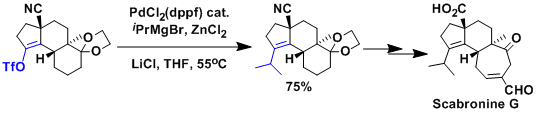
An example in the context of total synthesis of scabronine G.[1]
Newer systems in which secondary alkyl halides (for which β-hydride elimination is notoriously fast) can be used have been developed. An example shown below is one of the asymmetric versions reported by Fu.[2]
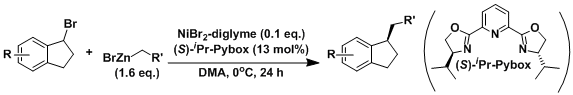
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Waters, S. P.; Tian, Y.; Li, Y.-M.; Danishefsky, S. J. J. Am. Chem. Soc. 2005, 127, 13514. DOI: 10.1021/ja055220x
[2] Arp, F. O.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 10482. DOI: 10.1021/ja053751f
-
Related Books
[amazonjs asin=”3527305181″ locale=”US” title=”Metal-Catalyzed Cross-Coupling Reactions (2 Volume Set)”]
[amazonjs asin=”3540421750″ locale=”US” title=”Cross-Coupling Reactions: A Practical Guide (Topics in Current Chemistry)”]
[amazonjs asin=”3642227481″ locale=”US” title=”Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis (Topics in Organometallic Chemistry)”]
[amazonjs asin=”0471972029″ locale=”US” title=”Palladium Reagents and Catalysts: Innovations in Organic Synthesis”]
[amazonjs asin=”0470850329″ locale=”US” title=”Palladium Reagents and Catalysts: New Perspectives for the 21st Century”]
[amazonjs asin=”0471315060″ locale=”US” title=”Handbook of Organopalladium Chemistry for Organic Synthesis (2 Vol. Set)”]

