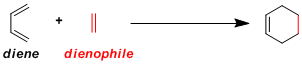- Generality
- Reagent availability
- Experimental User Friendliness
- Historical Significance
- Criteria #5
-
General Characteristics
-The most classic example of [4+2] cycloaddition reaction in which a diene and a dienophile combine to give a cyclohexene. The reaction generally proceeds with good stereo- and regioselectivity. With its exceptional practicality, Diels-Alder reaction is almost always the first choice when designing a synthesis for cyclic (especially six-membered ring) compounds.
–The Danishefsky’s diene is a synthetically useful reagent that provides better regioselectivity and reactivity than ordinary dienes. The Danishefsky-Brassard diene and the Rawal diene are the reagents along the same lines.
-Otto Diels and Kurt Alder shared the Nobel Prize in Chemistry in 1950 for the discovery and development of this important pericyclic reaction.
-
General References
・Diels, O.; Alder, K. Liebigs Ann. Chem. 1928, 460 , 98 DOI:10.1002/jlac.19284600106
・Kloetzel, M. C. Org. React. 1948, 4, 1.
・Holms, H. L. Org. React. 1948, 4, 60.
・Martin, J. G. Chem. Rev. 1961, 61, 537. DOI:10.1021/cr00013a013
・Sustman, R. et al. Angew. Chem. Int. Ed. Engl. 1980, 19, 779.
・Oppolzer, W. Comprehensive Organic Synthesis 1991, 5, 315.
・Roush, W. R. Comprehensive Organic Synthesis 1991, 5, 513.
・Yamabe, S.; Minato, T. J. Org. Chem. 2000, 65, 1830. DOI: 10.1021/jo9919310
・Nicolaou, K. C.; Synder, S. A.; Montagnon, T.; Vassilikogiannakis, G. Angew. Chem. Int. Ed. 2002, 41, 1668. [Abstract]
-
Reaction Mechanism
Normally, a diene containing an electron donating group (EDG) and a dienophile containing an electron withdrawing group (EWG) are used. This is because the combination minimizes the energy gap between HOMO of the diene and LUMO of the dienophile and the stabilizing interaction between the orbitals is maximized, hence the reactivity of cycloaddition (normal electron demand mode). According to the same frontier molecular orbital theory, the combination of a diene with EWG and a dienophile with EDG works also (inverse electron demand mode).

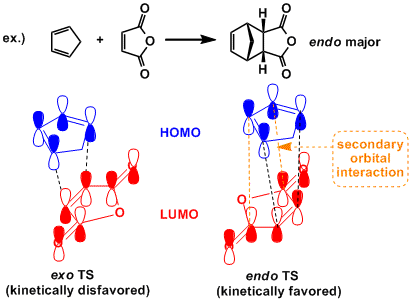
The reaction is concerted and endo products are formed preferentially (the endo rule). In normal electron demand reaction, this observation is generally explained by the secondary orbital interactions, but endo/exo selectivity is so largely influenced by steric factors that exo-selective cases are known depending on the substrates. In particular, in intramolecular Diels-Alder reactions where the ring rigidity restricts the conformational freedom of the molecule, the endo rule is often overridden.
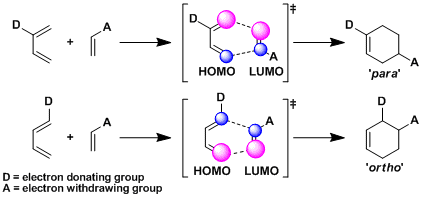
As simple electronic theory predicts, the substituents end up attached at ortho and para positions of the Diels-Alder adduct (the ortho-para rule). More precisely, the frontier orbital theory explains that cycloadditions proceed in such a way that allows the reacting atoms with large coefficients of HOMO and LUMO to overlap.
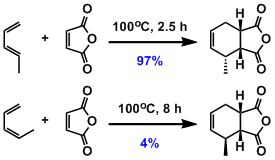
Dienes react in s-cis (cisoid) conformation in the cyclic transition state. Diels-Alder reaction cannot proceed with the diene in s-trans (transoid) conformation. For example, Z-1,3-pentadiene that tends not to assume s-cis conformation is far less reactive than the E-isomer.
-
Examples
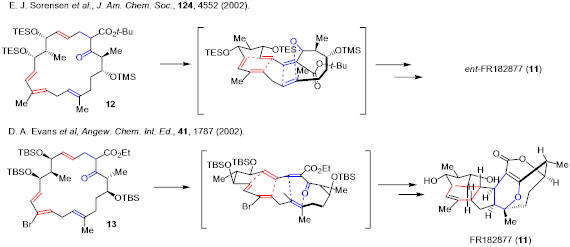
Diels-Alder reaction has been used in biomimetic total synthesis of natural products. For example, Sorensen’s and Evans’ groups each accomplished total synthesis of FR182877 based on intraannular Diels-Alder reaction as their key steps.
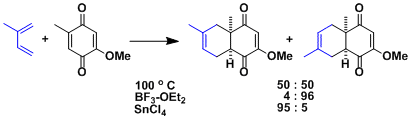
Lewis acids are known to influence the regioselectivity depending on their ability to chelate the substrates.
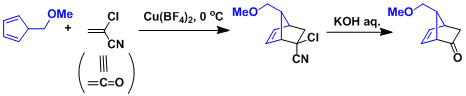
The synthesis of prostaglandins: α-Chloroacrylonitrile serves as a ketene equivalent.
-
Experimental Procedure
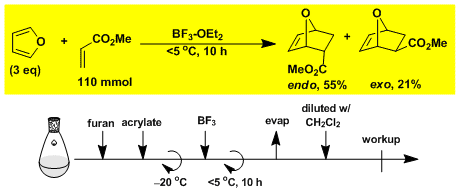
Diels-Alder reaction of furan and methyl acrylate.[1]
-
Experimental Tips
-
References
[1] Bull. Chem. Soc. Jpn. 1984, 57, 3339.
-
Related Books
[amazonjs asin=”0198792751″ locale=”US” title=”Organic Stereochemistry (Oxford Chemistry Primers)”]
[amazonjs asin=”0198503075″ locale=”US” title=”Pericyclic Reactions (Oxford Chemistry Primers)”]
[amazonjs asin=”0849378664″ locale=”US” title=”Lewis Acids and Selectivity in Organic Synthesis (Organic & Bio-organic Chemistry)”]
[amazonjs asin=”3527301593″ locale=”US” title=”Cycloaddition Reactions in Organic Synthesis”]
[amazonjs asin=”047180343X” locale=”US” title=”The Diels-Alder Reaction: Selected Practical Methods”]