- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
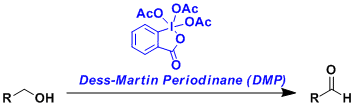
-The oxidation of alcohols by Dess-Martin periodinane (DMP).
-The reaction proceeds at room temperature, converting alcohols into aldehydes.
-Reactive and mild enough to be used for sterically hindered alcohols and α-substituted carbonyl compounds prone to racemization.
-Used frequently in the synthesis of complex molecules.
-Can be used for many acid- and base-sensitive compounds. For compounds that are sensitive to the slight acidity of the two equivalents of acetic acid produced by the reaction, DMP can be used with the addition of a buffer such as pyridine.
-
General References
・Dess, D. B.; Martin, J. C. J. Org. Chem. 1983, 48, 4155. DOI:10.1021/jo00170a070
・Dess, D. B.; Matrin, J. C. J. Am. Chem. Soc. 1991, 113, 7277. doi:10.1021/ja00019a027
・Stevenson, P. J.; Treacy, A. B. J. C. S. Perkin Trans. 2 1997, 589. DOI: 10.1039/a605253c
-
Reaction Mechanism
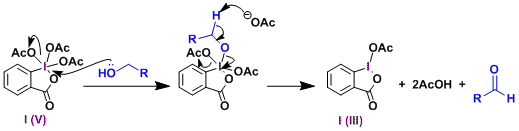
-
Examples
DMP is a highly functional group tolerant reagent as shown in the example below. It is used regularly in natural product synthesis. The addition of water occasionally shows beneficial effects.
Example 1.[1]
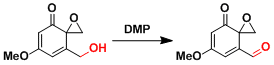
Example 2.[2]
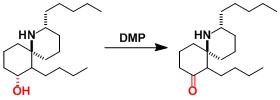
The synthesis of spongistatin 2.[3]
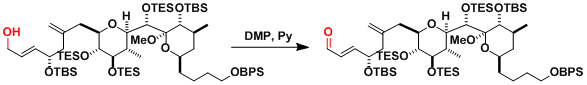
-
Experimental Procedure
The DMP reagent is readily prepared from 2-iodobenzoic acid. The preparation method reported in the original paper was somewhat irreproducible, but the modified method that uses catalytic TsOH solved the problem. The oxidation to IBX can be done reasonably safely and easily using Oxone.
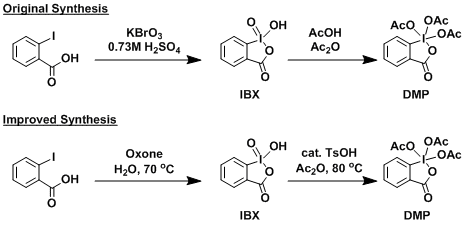
The reaction workup is very simple: The mixture can either be diluted with ether and washed with aqueous NaOH or NaHCO3/Na2SO3, or directly subjected to column purification.
-
Experimental Tips
DMP used to be unavailable commercially because of its explosive potential, but it is available today with the establishment of improved preparation procedures. Since it is a relatively expensive reagent, one needs to prepare it him/herself for a large scale experiment. In that case, note that the activity of the reagent tends to vary depending on the amount of residual water from the workup. DMP and its synthetic precursor (IBX) are hypervalent iodine compounds whose risks as explosives are well known, thus one must plan experimental scale carefully and handle the reagent with necessary caution.
-
References
[1] 大学院講義有機化学(Japanese book) II p.174
[2] ファルマシア (Japanese magazine) 1996, 32(9)
[3] Smith, A. B., III et al. Angew. Chem. Int. Ed. 2001, 40, 196. [abstract]
[4] (a) Dess, D.B.; Martin, J. C. J. Org. Chem. 1983, 48, 4155. (b) idem. J. Am. Chem. Soc. 1991, 113, 7277. doi:10.1021/ja00019a027 (c) Stevenson, P. J.; Treacy, A. B. J. C. S. Perkin Trans. 2 1997, 589.
[5] Irekand, R.E.; Liu, L. J. Org . Chem. 1993, 58, 2899. DOI: 10.1021/jo00062a040
[6] Frigerio, M.; Sntagostino, M.; Sputore, S. J. Org. Chem. 1999, 64, 4537. DOI: 10.1021/jo9824596
-
Related Books
[amazonjs asin=”3642079067″ locale=”US” title=”Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis (Topics in Current Chemistry)”]
[amazonjs asin=”0123909481″ locale=”US” title=”Hypervalent Iodine in Organic Synthesis”]