Overall Score5
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
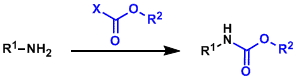
In order to tame the basicity and the high reactivity of amines during multi-step synthesis, it is common to protect them with electron-withdrawing functional groups. Carbamate meets this need well in terms of generality and the convenience of protection/deprotection, better than amide and sulfonamide. Carbamate protection is essential in peptide synthesis and used regularly in all other situations as well.
-
General References
-
Reaction Mechanism
-
Examples
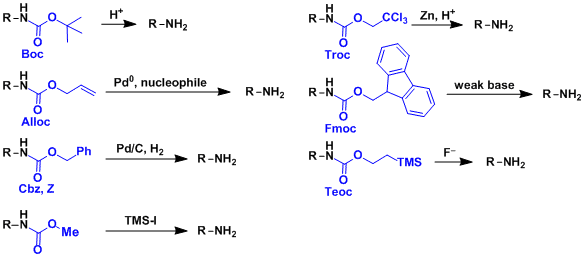
Commonly used carbamates are listed below. Each has its own deprotection conditions, allowing for orthogonal strategies.
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books
[amazonjs asin=”0471697540″ locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”]