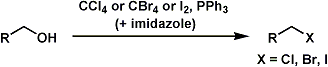- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The Appel reaction converts primary and secondary alcohols into corresponding alkyl halides. The reaction is suitable for both acid- and base-sensitive alcohols since the reaction conditions are neutral.
-
General References
・Downie, I.; Holmes, J.; Lee, J. Chem Ind. 1966, 22, 900.
・Calzada, J. G.; Hooz, J. Org. Synth. 1974, 54, 63. [PDF]
・Appel, R. Angew. Chem. Int. Ed. 1975, 14, 801. doi:10.1002/anie.197508011
・van Kalkeren, H. A.; van Delft, F. L.; Rutjes, F. P. J. T. Pure Appl. Chem. 2013, 85, 817. doi:10.1351/PAC-CON-12-06-13
-
Reaction Mechanism
The reaction is driven based on the high affinity between phosphorus and oxygen.
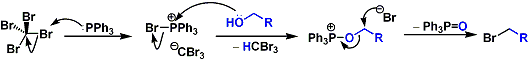
-
Examples
The substitution involves the inversion of stereochemistry. [1]
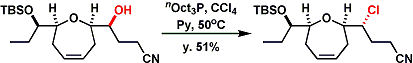
The Appel reaction proceeds at very low temperatures under reactive conditions using hexachloroacetone[2a] or hexabromoacetone.[2b] These can be used for even some tertiary alcohols including adamantanol.
Asymmetric phosphine oxidation under the Appel conditions. [3]
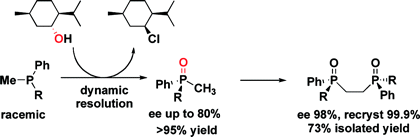
Modified conditions in which the phosphine can be used catalytically have been developed recently.[4]
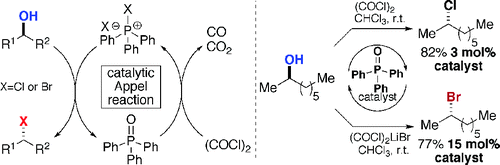
-
Experimental Procedure
The synthesis of geranyl chloride.[4]
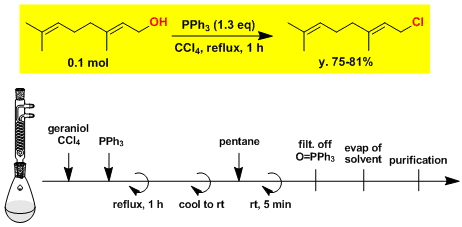
-
Experimental Tips
-Bromination with CBr4 proceeds at 0˚C~room temperature, but chlorination with CCl4 requires reflux temperature.
-The separation of phosphine oxide from the product can be a hassle. An alternative strategy to effect the same transformation would be the Finkelstein reaction, which is a two-step reaction involving mesylation followed by halide displacement.
-
References
[1] Suzuki T. et al. Tetrahedron Lett. 2001, 42, 65. doi:10.1016/S0040-4039(00)01880-3
[2] (a) Magid, M. R.; Fruchey, S.; Johnson, W. L.; Allen, T. G. J. Org. Chem. 1979, 44, 359. DOI: 10.1021/jo01317a011 (b) Tongkatea, P.; Pluempanupata, W.; Chavasiri, W. Tetrahedron Lett. 2008, 49, 1146. doi:10.1016/j.tetlet.2007.12.061
[3] Bergin, E.; O’Connor, C. T.; Robinson, S. B.; McGarrigle, E. B.; O’Mahony, C. P.; Gilheany, D. G. J. Am. Chem. Soc. 2007, 129, 9566. DOI:10.1021/ja072925l
[4] (a) Denton, R.; An, J.; Adeniran, B.; Blake, A.; Lewis, W.; Poulton, A. J. Org. Chem. 2011, 76, 6749. doi:10.1021/jo201085r (b) van Kalkeren, H. A.; Leenders, S. H. A. M.; Hommersom, C. A.; Rutjes, F. P. J. T.; van Delft, F. L. Chem. Eur. J. 2011, 17, 11290. DOI: 10.1002/chem.201101563
[5] Calzada, J. G.; Hooz, J. Org. Synth. 1974, 54, 63. [PDF]
-
Related Books