- Generality
- Reagent Availability
- Experimental User Friendliness
- Scope of Reported Examples
- Criteria #5
-
General Characteristics
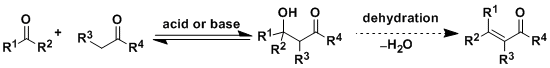
-In aldol reactions, the enol or the enolate of a ketone attacks an aldehyde or another ketone to generate a β-hydroxyketone (“aldol”). In the most classical procedure, the reaction is carried out with heating and either a Brönsted acid or a base.
-Every step of aldol reaction is governed by equilibrium and reversible. The reverse reaction is called retro-aldol reaction. The main products are determined by thermodynamic stability and the yields are largely dependent on the type of substrates. Precise control of stereochemistry is usually difficult. The formation of various byproducts (elimination product, self-condensation product, etc.) is also a common problem therefore achieving high chemoselectivity requires skills and creativity.
-Dehydration of the aldol intermediate by treating it with either acid or heat gives an α,β-unsaturated ketone (aldol condensation).
-
General References
・Kane, R. J. Prakt. Chem. 1838, 15, 129.
・Kane, R. Ann. Phys. Chem. Ser. 2 1838, 44, 475.
・Heathcock, C. H. Comprehensive Organic Synthesis 1991, 133.
・Mahrwald, R. ed. Modern Aldol Reactions Wiley-VCH, 2004
・Review: Palomo, C.; Oiarbide, M.; Garcia, J. M. Chem. Eur. J. 2002, 8, 36. [abstract]
・Review: Palomo, C.; Oiarbide, M.; Garcia, J. M. Chem. Soc. Rev. 2004, 33, 65. DOI: 10.1039/b202901d
・Review: Schetter, B.; Mahrwald, R. Angew. Chem. Int. Ed. 2006, 45, 7506. doi:10.1002/anie.200602780
-
Reaction Mechanism
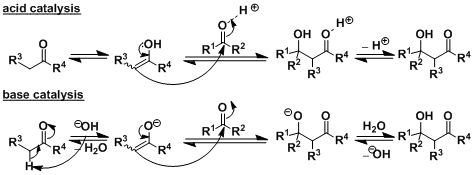
-
Examples
Stereocontrolled direct crossed aldol reactions without pre-formation of silyl enol ethers would be significant in terms of atom economy and extremely useful. Shibasaki developed the first catalytic asymmetric direct aldol reaction using the lanthanide-alkali metal hetero-bimetallic catalyst.[1]
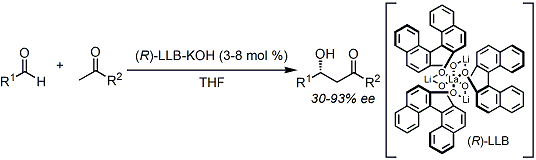
Proline-catalyzed direct asymmetric aldol reactions reported by List and Barbas[2] triggered the explosive development of organocatalysis research (the List-Barbas aldol reaction).

-
Experimental Procedure
-
Experimental Tips
-
References
[1] (a) Yamada,Y. M. A.; Yoshikawa, N.; Sasai, H.; Shibasaki, M. Angew. Chem. Int. Ed. Engl. 1997, 36, 1871. doi:10.1002/anie.199718711 (b) Yoshikawa, N.; Yamada, Y. M. A.; Das, J.; Sasai, H.; Shibasaki, M. J. Am. Chem. Soc. 1999, 121, 4168. DOI: 10.1021/ja990031y
[2] (a) List, B.; Lerner, R. A.; Barbas, C. F., III J. Am. Chem. Soc. 2000, 122, 2395. DOI: 10.1021/ja994280y (b) Notz, W.; List, B. J. Am. Chem. Soc. 2000, 122, 7386. DOI: 10.1021/ja001460v (c) List, B.; Pojarliev, P.; Castello, C. Org. Lett. 2001, 3, 573. DOI: 10.1021/ol006976y (d) Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C. F., III J. Am. Chem. Soc. 2001, 123, 5260. DOI: 10.1021/ja010037z
-
Related Books
[amazonjs asin=”3527307141″ locale=”US” title=”Modern Aldol Reactions (2 Volume Set)”]